(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a presentation by Dr. Marina Parry discussing an ancillary study of the STAMPEDE trial assessing clinical qualification of transcriptome signatures for advanced prostate cancer starting ADT with or without abiraterone acetate and prednisolone. The addition of abiraterone acetate and prednisolone to ADT is standard of care for advanced prostate cancer (metastatic, M1 and high-risk non-metastatic, M0). Transcriptome classifiers are used in several cancer types to stratify treatment but not for advanced prostate cancer, which may be a missed opportunity for patient stratification. Dr. Parry and colleagues tested transcriptome signatures as prognostic and predictive biomarkers for patients starting ADT +/- abiraterone acetate and prednisolone.
Whole transcriptome profiling was performed using a clinical test (DECIPHER) on tumor index core mRNA from patients randomized 1:1 ADT vs ADT + abiraterone acetate and prednisolone in STAMPEDE:
Dr. Parry and colleagues selected 58 signatures for association with outcome, including four signatures shown to be prognostic in previous studies: AR-A, DECIPHER Genomic Classifier, PAM50, and PSC. Additionally, there were 54 signatures included capturing pathways of importance in prostate cancer biology (ie. AR biology, immune signaling, cell cycle pathways, and cell of origin). A pre-specified statistical analysis plan was approved by the trial oversight groups. The primary objectives were to (i) establish the DECIPHER genomic classifier as a prognostic marker for OS in M1 patients and MFS in M0 patients, and (ii) test the ability of AR-A, PAM50, PSC, and the DECIPHER genomic classifier to predict the treatment effect of abiraterone + prednisolone for OS. Cox models were fit with mRNA signature, abiraterone acetate and prednisolone (+/-), age, WHO performance status, pre-ADT PSA, NSAIDs/aspirin use, Gleason score, and disease burden (M0N0 vs M0N1 vs M1 low volume vs M1 high volume) as covariates. Primary analyses included DECIPHER genomic classifier (continuous) for prognosis and AR-A (average vs low) for prediction. This trial closed to follow up in November 2021 with the data cleaned and locked on July 3, 2022.
Of 1,917 patients (full trial cohort) enrolled from November 2011 - January 2014, 1,824 consented to tumor analysis. 1,298 (71%) were reviewed centrally, 831 (64%) gave transcriptomes, and 781 (94%) passed quality control. Of the 781, 50% vs 52% in full trial cohort were M1; other clinical variables, well balanced. The median follow-up was 94 months (IQR: 84 - 97). Neither AR-A, PAM50, PSC or the DECIPHER genomic classifier were predictive of treatment effect of abiraterone acetate + prednisolone on OS in the combined cohort (M0 and M1):

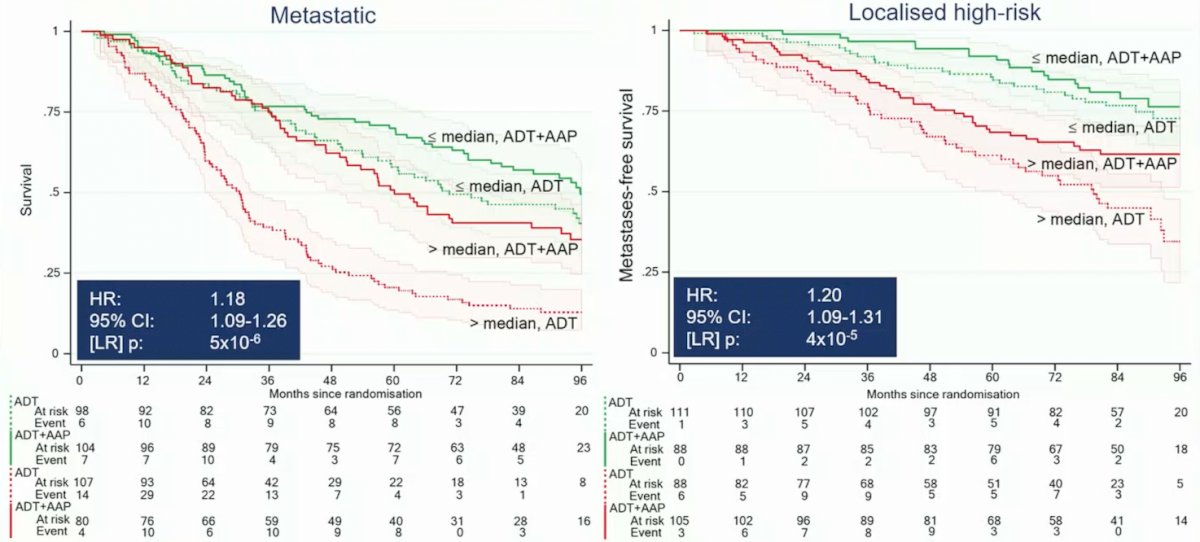
However, the genomic classifier was strongly prognostic in advanced prostate cancer (per 0.1 increment, M1 OS (HR 1.18, 95% CI 1.09 - 1.26, p < 0.001), M0 MFS (HR 1.20, 95% CI 1.09 - 1.31, p < 0.001):
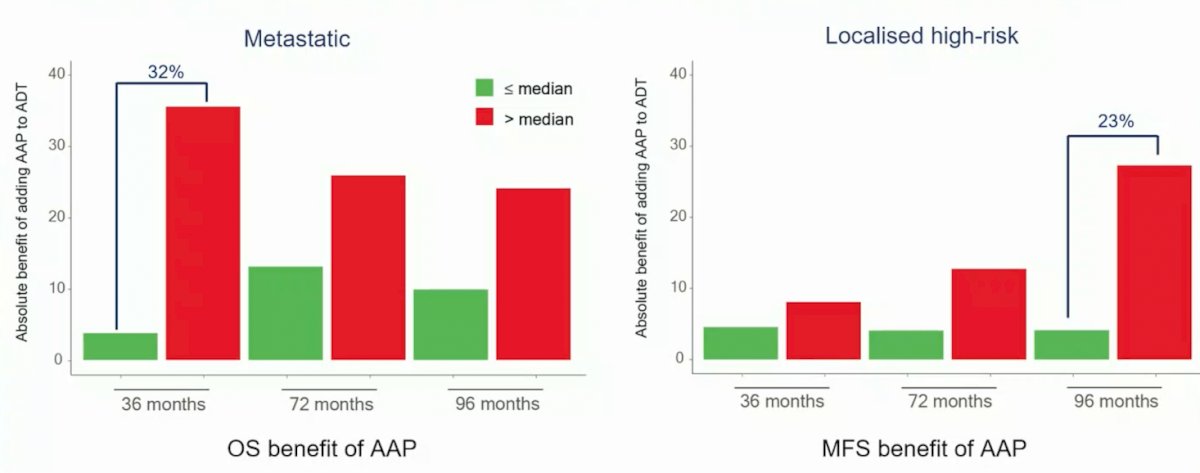
As follows is the absolute benefit of adding abiraterone acetate + prednisolone to ADT, which varies by DECIPHER score:
Additionally, all primary signatures were prognostic in M0 for MFS, but only the DECIPHER genomic classifier was prognostic across all endpoints in both M0 and M1 cohorts:

Prognostic signatures are also different in M0 and M1 patients, with the following showing testing of 54 signatures for association with outcome (MFS in M0 and OS in M1, p < 0.001):
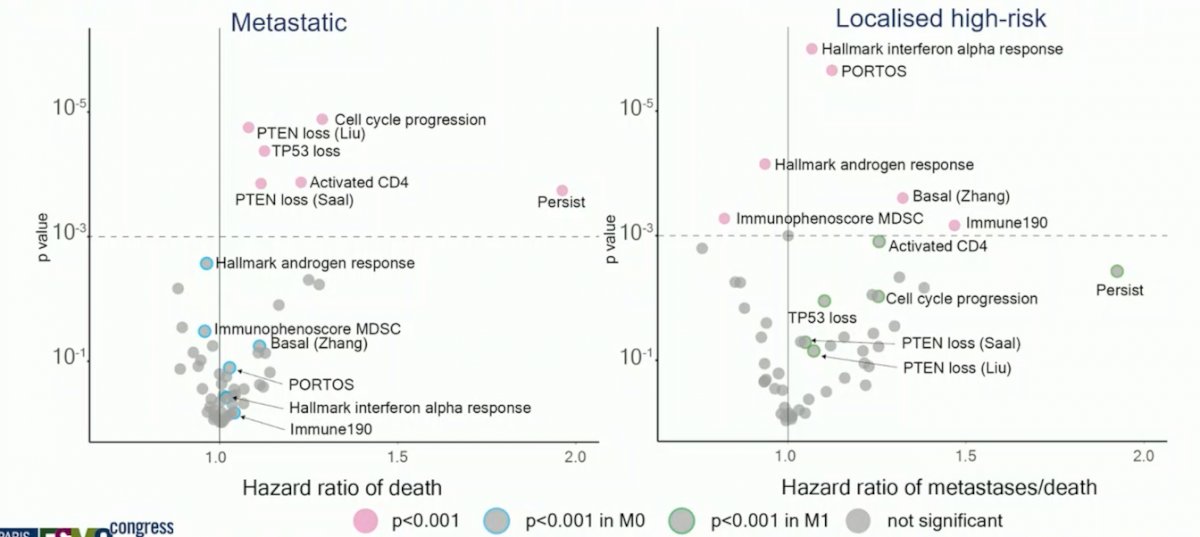
Finally, there was significant interaction with metastatic stage observed, specifically with regards to three immune microenvironment signatures: (i) the Hallmark interferon alpha response (p = 0.0001), (ii) PORTOS (p = 0.0001), and (iii) Immune190 (p = 0.007).
Dr. Parry concluded her presentation by discussing an ancillary study of the STAMPEDE trial assessing clinical qualification of transcriptome signatures for advanced prostate cancer starting ADT with or without abiraterone acetate and prednisolone with the following take-home messages:
- There was a consistent effect of the addition of abiraterone acetate + prednisolone on OS in the combined cohort
- The absolute benefit of adding abiraterone acetate + prednisolone to ADT was greater in patients with higher DECIPHER genomic classifier risk tumors, which has potential clinical utility, especially in localized disease
- All four primary signatures were prognostic in M0 for MFS, with the DECIPHER genomic classifier prognostic in both M0 and M1 patients across all endpoints
- Exploratory analysis reveals different signatures are prognostic in M1 and M0, with an interaction with metastatic stage observed in immune related signatures
Presented by: Marina Parry, BSc, PhD, Senior Research Fellow, Oncology Department - Treatment Resistance, UCL Cancer Institute London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


