He began by discussing the work presented by Dr. Groeneveld. He noted that molecular subtypes of bladder cancer has been recognized for some time beginning with work from MD Anderson and using The Cancer Genome Atlas in 2014 which noted that this may be prognostic for patient outcomes. One of the first observations was that the basal subtype did poorly overall but responded well to chemotherapy. Following a number of disparate groups’ work, there was an amalgamation of the subtype approach into a consensus approach with four molecular subtypes including basal, claudin-low, luminal-infiltrated, and luminal.
Work from Dr. Seiler and colleagues demonstrated that there was both prognostic value to these, as well as predictive value with patients having the basal signature responding particularly well to neoadjuvant chemotherapy.
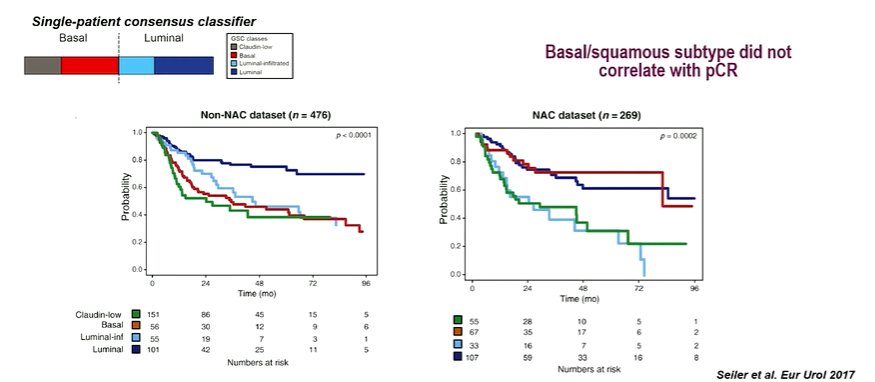
Interesting, despite these improvements in surgical, there was not a strong correlation with pathological complete response rates.
Subsequent follow-up of the TCGA work suggested an alternative approach in which five molecular subtypes were now defined, including the newly added neuronal subtype. Ongoing work in 2020 subsequently derived a classification schema with 6 molecular subtypes. He noted that these subtypes are all defined on the basis of gene expression profiles. There is some prognostic value to these, however, there is some uncertainty regarding clinical implications.
He noted that there is an immediate benefit to the use of molecular subtypes in that they may be applied to one’s own dataset relatively easily. In doing so, he and his group found a relatively poor linkage, particularly among the luminal subtypes, which may relatively easily fall into multiple differing categories.
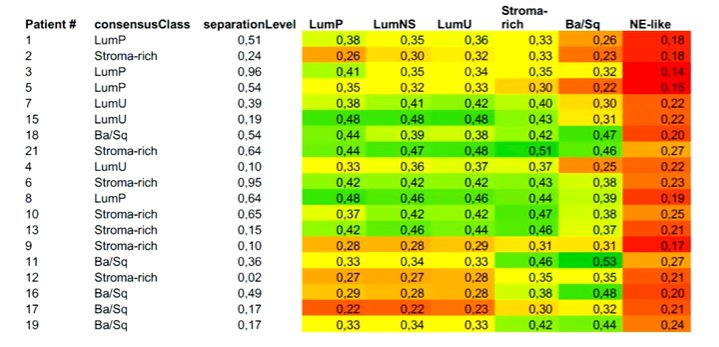
Highlighting the work from Dr. Groeneveld based on the VESPER trial, he noted that this study was restricted to the NAC subset of the VESPER trial. The authors performed molecular subtyping based on tissue following macrodissection, finding that heterogeneity on immunohistochemistry was associated with mixed molecular subtyping. He noted that a substantial proportion of the mixed tumors were from disparate molecular subtypes.
The authors demonstrated that molecular subtype was associated with the response to NAC, with a lower response rate seen in those with a mixed subtype. Interestingly, in this cohort, the basal/squamous subset had a relatively moderate response to NAC and poor prognosis, a finding that contrasts the previous observation from Dr. Seiler.
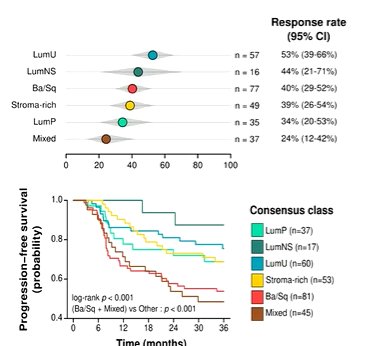
Summarizing this article, he noted that not all heterogeneity is significant or clinically meaningful. However, patients with basal/squamous and mixed subtype tumors, as well as those with lymphovascular invasion have worse outcome than other subtypes after NAC. This somewhat contradicts prior data and leaves some unanswered questions. As a result, there is a somewhat unclear path forward. However, the few “clear-cut” clinical associations, it may be necessary to reduce subtypes to define clinical relevance.
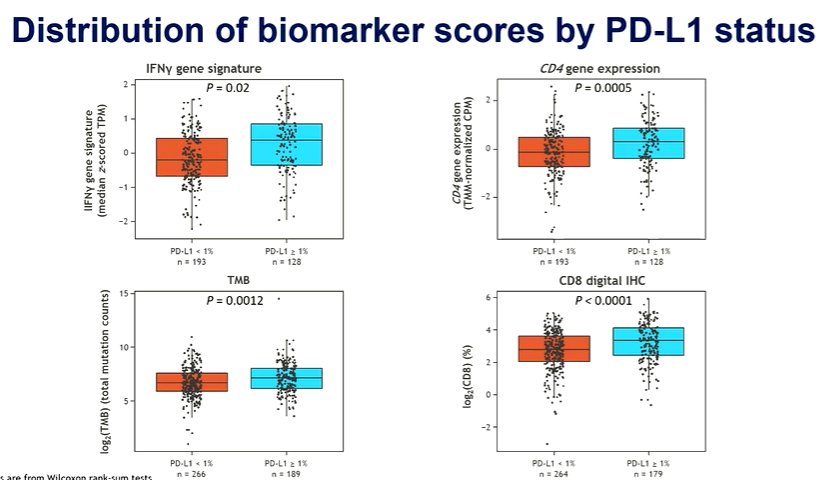
He then moved to abstract 1737MO in which Dr Neechi presented a biomarker analysis of the CheckMate 274 trial of adjuvant nivolumab in resected, muscle invasive urothelial carcinoma. The primary report of this study showed that adjuvant nivolumab improves disease-free survival, with a particularly large effect in patients with PD-L1 expression of 1% or greater. Currently, overall survival data are awaited. In this analysis, the authors used a subset of patients with available tissue for exploratory biomarker analyses and assess CD8, tumor mutational burden (TMB), CD4 expression, and IFNgamma signatures. The pro-inflammatory markers (including IFNgamma and CD4) both predicted response to nivolumab, with a greater benefit seen among patients with higher expression levels.
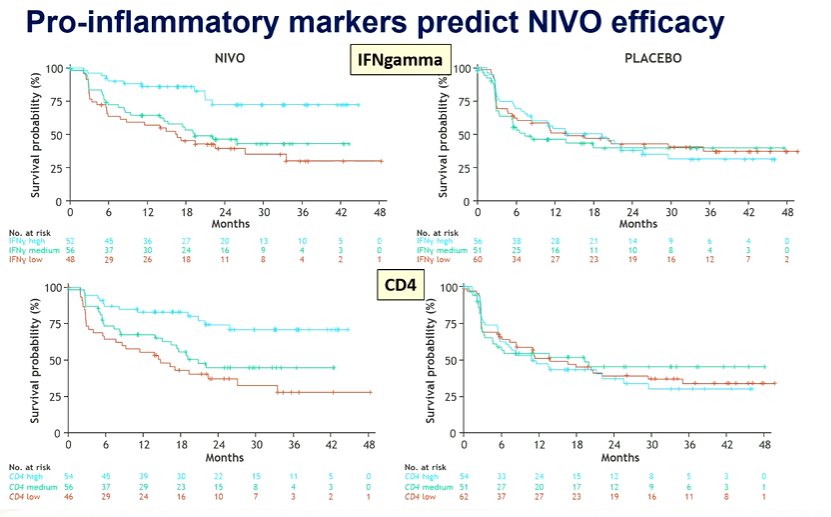
He emphasized that the authors used PD-L1 status for stratification. Interestingly, these pro-inflammatory markers were somewhat higher in the PD-L1 positive population though the ranges overlapped heavily.
Moving forward, he suggested that these findings confirm that pre-existing immunity are predictive for immune checkpoint inhibitor monotherapy. However, the question remains how we may improve outcomes of these agents for patients with low pre-existing immunity. One of the ways to potentially do this would be to use the therapy in the neoadjuvant setting. In the context of melanoma, neoadjuvant therapy provides a much greater response than adjuvant treatment.
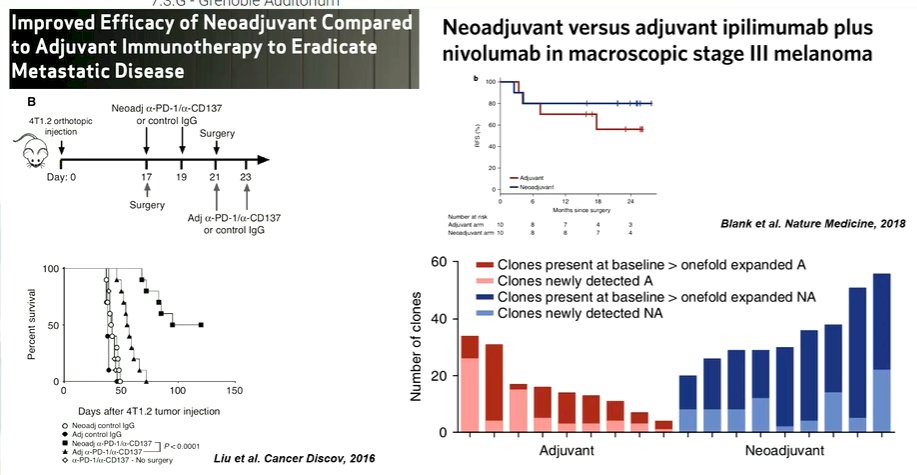
This is also supported by murine models. However, in available data thus far, the neoadjuvant response of immune checkpoint inhibitor monotherapy is relatively restricted to “hot” tumors. To improve neoadjuvant response, Dr. Van der Heijden postulated that we may perhaps use chemotherapy to improve response in immunologically cold tumors. This has worked in other tumor sites though combined chemoimmunotherapy was not efficacious in the first-line metastatic bladder cancer space. Alternatively, he suggested that priming may be increased by adding the anti-CTLA-4 agent ipilimumab to nivolumab.
Additionally, the introduction of new drugs (including antibody-drug conjugates and FGFR inhibitors) will change the treatment landscape further.
Presented by: Michiel S. Van der Heijden, MD, PhD, Netherlands Cancer Institute, Amsterdam, NetherlandsWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.


