(UroToday.com) In the Urothelial Cancer poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Grivas presented a poster based on results from the PREVAIL Prospective Cohort Study assessing the prevalence of Programmed death-ligand 1 (PD-L1) high expression in patients with advanced urothelial carcinoma. Across a number of studies, PD-L1 expression in aUC has been studied extensively in clinical trials. The prevalence of high PD-L1 expression has been somewhat variable, with 60% of patients in the DANUBE trial having high tumor PD-L1 expression using the Ventana PD-L1 (SP263) Assay. To better understand testing practices and PD-L1 landscape in the real-world, studies outside of a trial context are required.
To assess this, the authors used the PREVAIL (NCT03788746) which enrolled patients with locally advanced/metastatic UC undergoing 1L therapy at community practice sites who had pre-treatment tumor tissue available for central PD-L1 testing with the Ventana PD-L1 (SP263) Assay.
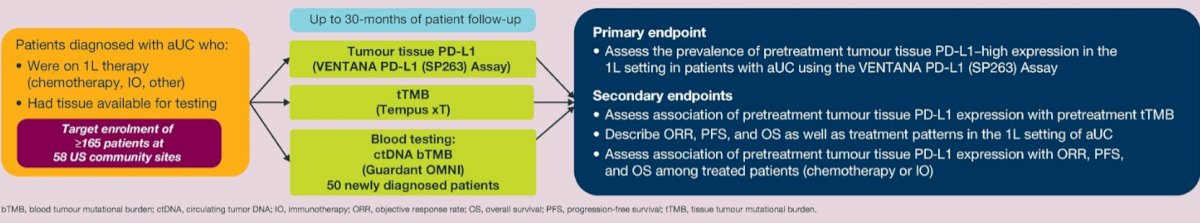
The primary endpoint was the prevalence of pre-treatment tumor PD-L1 high expression, defined as ≥25% of tumor cells (TCs) exhibiting membranous staining or, immune cells present (ICP) >1% and immune cells with staining (IC+) ≥25% or, ICP=1% and IC+ =100%.
The authors identified 129 evaluable patients. Among these patients, the median age was 72 years and 66% were men. The vast majority were White (86%) and ECOG PS 0-1 (85%). In terms of disease location, 25% had upper tract tumor, and 35% visceral metastases. One quarter (25%) were never smokers.
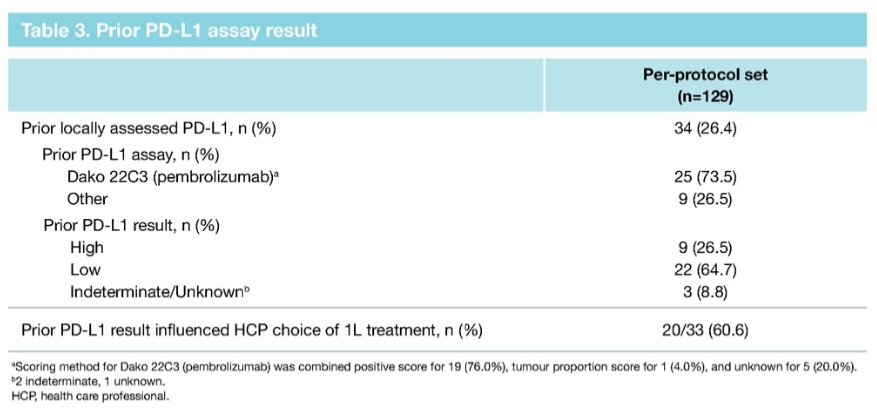
Prior to entry into the PREVAIL study, only 33 (26%) patients had prior PD-L1 testing.
In terms of pre-treatment PD-L1 expression, this was high in 71 (55%) patients in the PREVAIL cohort. This is relatively comparable to findings in phase 3 clinical trials suggesting that those trial populations may be representative, at least in terms of PD-L1 expression, of the greater patient population.
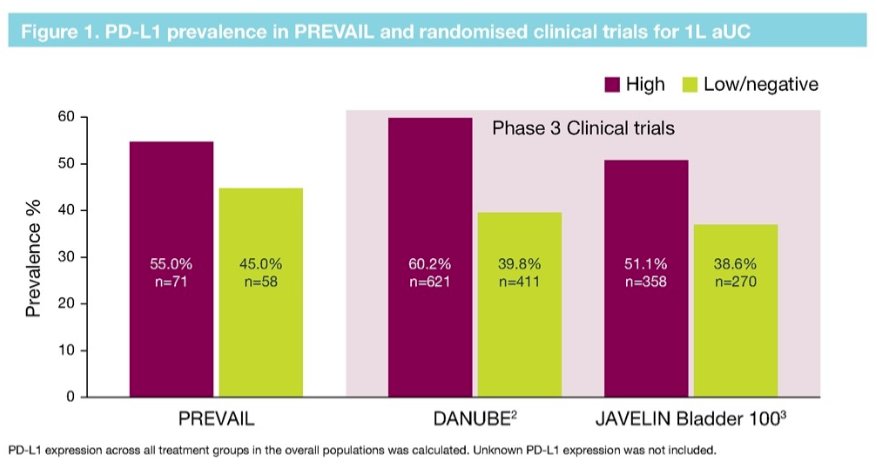
Thus, Dr. Grivas concluded that real-world data suggest a similarly high prevalence of PD-L1 high expression (55%) in patients starting first-line systemic therapy for advanced urothelial carcinoma as has been observed in clinical trials. However, only a quarter of patients had prior PD-L1 testing, which limited inter-assay concordance evaluation.
Presented by: Petros Grivas, MD, Ph.D., Professor, Clinical Director of Genitourinary Cancers Program, University of Washington, Associate Member, Clinical Research Division, Fred Hutchinson Cancer Research Center.
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


