(UroToday.com) The Earlier Treatment in Prostate Cancer “How can we maximize the therapeutic index?” educational session at the European Society of Medical Oncology’s (ESMO) 2021 congress included a presentation by Nicolas Mottet discussing evidence-based treatment options in biochemically relapsed prostate cancer. Dr. Mottet notes that the definition of relapse after external beam radiotherapy is the nadir + 2 ng/mL, with the rationale for this being that it was the best definition to predict further metastases. It was initially based on the Phoenix consensus but later was considered as the main clinical definition of relapse. With regards to relapse after radical prostatectomy, Dr. Mottet notes that there is a major difference between PSA relapse (any PSA rise following an undetectable level) and a clinically significant PSA rise, which is the best predictor of further metastases. Post-radical prostatectomy, the definition of relapse is no longer a PSA of 0.2 ng/mL and rising. Currently, following an undetectable PSA (<0.1 ng/mL), the best PSA threshold to define a relapse is 0.4 ng/mL and rising (since 2018 EAU-ESTRO-SIOG-ESUR guideline), given that this is the best correlation with systemic progression; this definition has also been adapted as a consensus statement by ASCO. The threshold of PSA > 0.4 ng/mL and rising is the most clinically relevant threshold, but it is not the threshold to define relapse, and it is not the threshold to consider salvage treatment.
When the PSA is rising, a bone scan has a <1% positivity rate if the PSA is <10 ng/mL and is only useful when the PSA is >20 ng/mL or the patient has bone pain (or when PSA doubling time is < 6 months). Abdomino-pelvic MRI has a low sensitivity when the PSA is < 2 ng/mL, and whole-body MRI is inferior to PET PSMA. At a mean PSA of 2 ng/mL the percent positivity for PSMA is 71% and for MRI is 39%. For choline PET scan, the positivity rate is only 5-24% if the PSA is <1 ng/mL and up to 67-100% if the PSA is >5 ng/mL. Fluciclovine PET scan has better sensitivity compared to choline, with a sensitivity of ~50% when the PSA is <1 ng/mL. We now have data for 68Ga-PSMA-PET in the detection of relapse after radical prostatectomy, which has shown an overall detection rate of 53.6% for PSA 0.5-1.0 ng/mL and up to 71.3% for PSA 1-2 ng/mL. Of note, even with PSMA-PET, 62.1% of PET scans are negative at a PSA of <0.5 ng/mL. The recently published CONDOR trial focused on patients with biochemically recurrent disease, with a rising PSA after definitive therapy and negative or equivocal standard of care imaging (e.g., CT/MRI, bone scintigraphy, or F-18 fluciclovine).1 In this study, PyL-PET/CT correctly localized lesions in 84.8-87.0% of cases among three readers (lower bound of 95% CI: 77.8%-80.4%) against the composite standard of truth. The detection rate stratified by PSA-level is as follows:
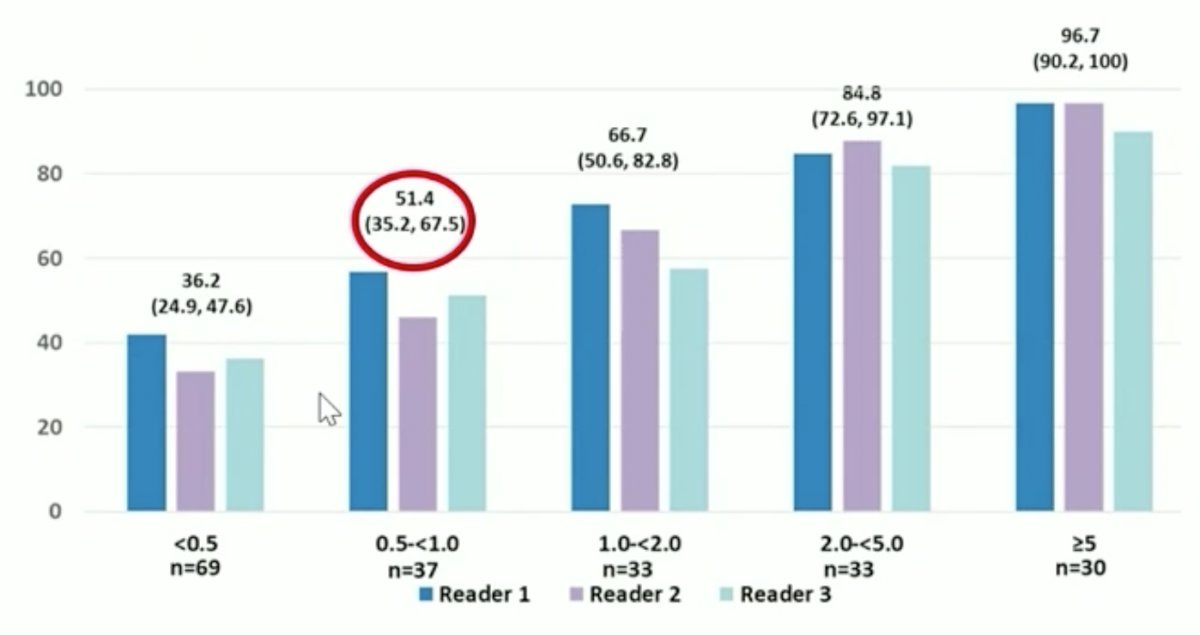
Similar to 68Ga-PSMA PET, DCFPyL PET scans were negative in 65.8% of cases at a PSA <0.5 ng/mL. The EAU guidelines2,3 state that in the setting of PSA recurrence after radical prostatectomy a PSMA PET-CT may be obtained if the PSA level is > 0.2 ng/mL and if the results will influence subsequent treatment decisions (level of evidence 2b, strength rating: weak). In cases where PSMA PET/CT is not available, and the PSA level is >= 1 ng/mL, clinicians should perform a fluciclovine PET/CT or choline PET/CT if the results will influence subsequent treatment decisions (strength rating: weak).
Dr. Mottet notes that relapse is important in that it is linked with metastasis free-survival, disease-specific survival, and overall survival. However, the impact of relapse may be highly variable given that across studies hazard ratios range from 1.03 to 2.32 with regards to survival outcomes. In a systematic review assessing the prognostic value of biochemical recurrence following treatment with curative intent, Van den Broeck et al.4 define the EAU low-risk biochemical recurrence as a PSA-doubling time >1 year and pathologic ISUP grade <4 (whereby salvage treatment should be discussed but may not be needed), and EAU high-risk biochemical recurrence as a PSA-doubling time <= 1 year or pathological ISUP grade 4-5 (whereby salvage treatment is needed).
When considering salvage radiotherapy after radical prostatectomy the RTOG 9601 trial assessed the utility of concomitant ADT.5 This phase III randomized clinical trial assessed bicalutamide monotherapy among salvage EBRT patients randomized to bicalutamide 150 mg PO daily vs placebo for 24 months. Overall survival rates at 12 years were 76.3% for patients taking bicalutamide compared to 71.3 taking placebo (p=0.04). Prostate cancer survival rates at 12 years were 5.8% for bicalutamide compared to 13.4% for placebo (p<0.001). The EAU guideline recommendations for local salvage treatment for biochemical recurrence after radical prostatectomy suggest that clinicians should offer PSA monitoring to patients with biochemical recurrence with low-risk features at relapse who may not benefit from intervention (strength rating: weak). Clinicians should offer salvage radiotherapy to patients with a PSA rise from an undetectable range, and once the decision is made to offer salvage radiotherapy, it should be given (at least 66 Gy) as soon as possible (strength rating: strong). Finally, the guidelines suggest that clinicians should offer hormonal therapy, in addition, to salvage radiotherapy to men with biochemical recurrence (strength rating: weak). Dr. Mottet also added that clinicians should not wait for a positive PET if salvage EBRT is planned and that salvage EBRT must be at least 66 Gy and may go up to 72 Gy.
Salvage therapy after external beam radiotherapy is less clear as we do not have randomized data to guide treatment. Most data for patients undergoing salvage radical prostatectomy, with other potential modalities of treatment being salvage cryotherapy or HIFU. Dr. Mottet emphasizes that local salvage therapy is possible and that re-irradiation is possible and safe in select circumstances. Furthermore, it is important to note that no local treatment has been consistently associated with improved outcomes and that there are variations in toxicity. There are major limitations with these studies given that the sample sizes are highly variable, most studies are uncontrolled single-arm, and there is heterogeneity of definitions for core outcomes. The EAU guidelines state that for salvage local therapy after external beam radiotherapy, clinicians should offer to monitor, including PSA to EAU low-risk BCR patients (weak), and only offer salvage radical prostatectomy, brachytherapy, HIFU, or cryoablation to highly selected patients with biopsy-proven local recurrence within a clinical trial setting or well-designed prospective cohort study (strong).
Dr. Mottet then discussed ADT for systemic relapse. Data from the CaPSURE database assessing 2,096 patients undergoing immediate versus deferred ADT (deferred until metastases, symptoms, PSA doubling time <12 months for a PSA >= 10 ng/mL or PSA doubling time <= 6 months).6 The mean time from primary treatment to PSA relapse was 37.4 (SD 34.2) months, and the mean follow-up from primary treatment was 91.4 (SD 48.4) months. The adjusted mortality hazard ratio for immediate versus deferred ADT was 0.91 (95% CI, 0.52-1.60), which translates to a similar 5-year survival (difference between groups: -2.0% (95% CI: -10.0 to 5.9%):

The TOAD study also assessed time of ADT in a prospective randomized fashion and found that immediate ADT was associated with a higher 5-year overall survival than delayed (>2 years) ADT (91% vs 86%) in patients with PSA relapse following predominate radiotherapy. Dr. Mottet states that the evidence for salvage ADT is weak at best. According to the EAU guidelines, clinicians should discuss deferred ADT with well-informed patients that are M1 and asymptomatic (weak), and that they should discuss combination therapy including ADT plus systemic therapy with all M1 patients (strong).
To conclude his presentation, Dr. Mottet discussed salvage lymph node dissection in the setting of isolated recurrence. Long-term outcomes of patients treated with salvage lymph node dissection for nodal recurrence until recently were essentially unknown. Bravi et al.7 undertook a multi-institutional approach to assess these long-term outcomes, including 189 patients who experienced PSA rise and nodal-only recurrence after radical prostatectomy and underwent salvage lymph node dissection at 11 centers between 2002 and 2011. Recurrences were detected with either 11C-choline or 68Ga PSMA. The primary outcome was cancer-specific mortality, and the secondary outcomes were overall mortality, clinical recurrence, biochemical recurrence, and ADT-free survival after salvage lymph node dissection. There were 110 and 163 patients who experienced clinical recurrence and biochemical recurrence, respectively, with clinical recurrence-free and biochemical recurrence-free survival at 10 years of 31% and 11%, respectively. After salvage lymph node dissection, a total of 145 patients received ADT, with a median time to ADT of 41 months:

Importantly, additional therapy was warranted in >60% of patients within 6 months of salvage lymph node dissection. At a median follow-up for survivors of 87 (IQR 51 to 104) months, 48 patients died, of which 45 died from prostate cancer. At multivariable analyses, patients who had PSA response after salvage lymph node dissection (HR 0.45; p = 0.001), and those receiving ADT within 6 months from salvage lymph node dissection (HR 0.51; p = 0.010) had a lower risk of death from prostate cancer.
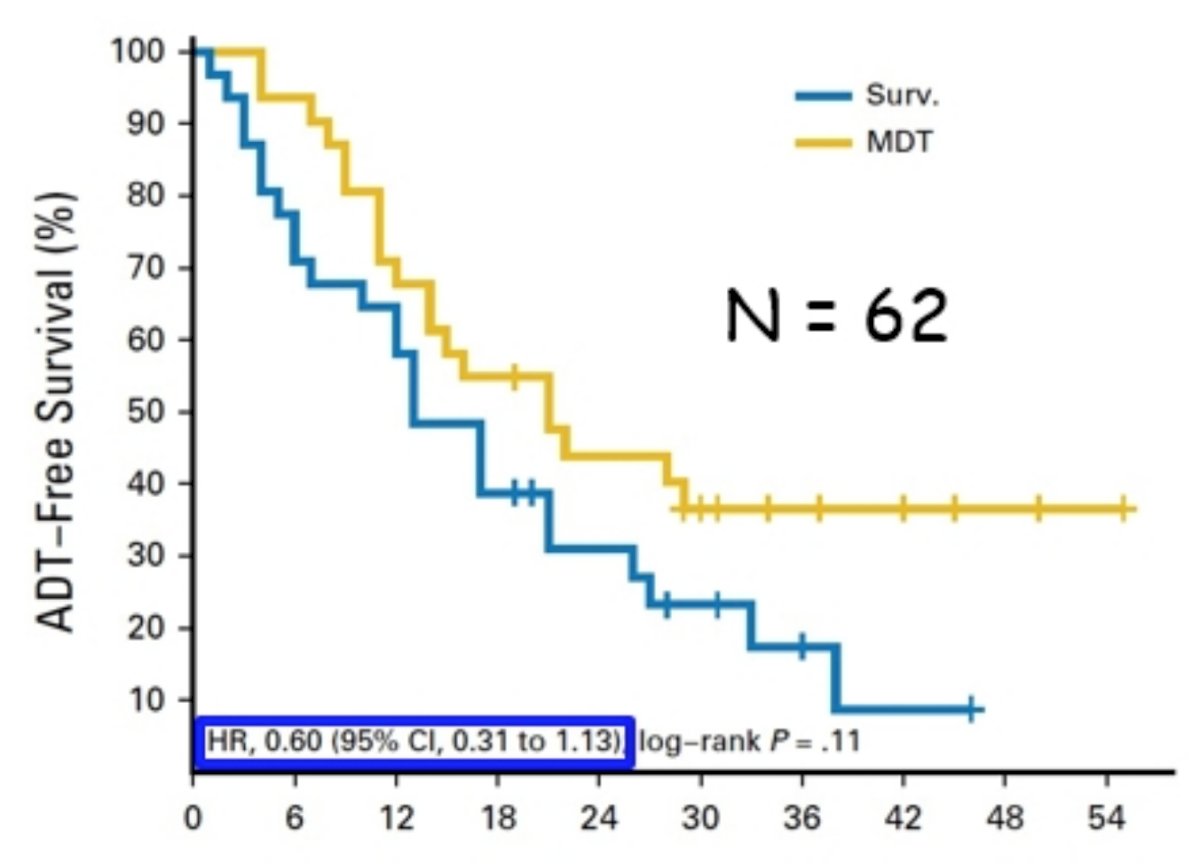
Treatment in the oligometastatic setting is also unclear, as there is only phase 2 data assessing targeted treatment of oligometastatic disease.8 Among 62 patients randomized, at a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI, 12 to 17 months) for the surveillance group and 21 months (80% CI, 14 to 29 months) for the metastasis directed therapy group (HR 0.60, 95% CI 0.31-1.13, log-rank p = 0.11):
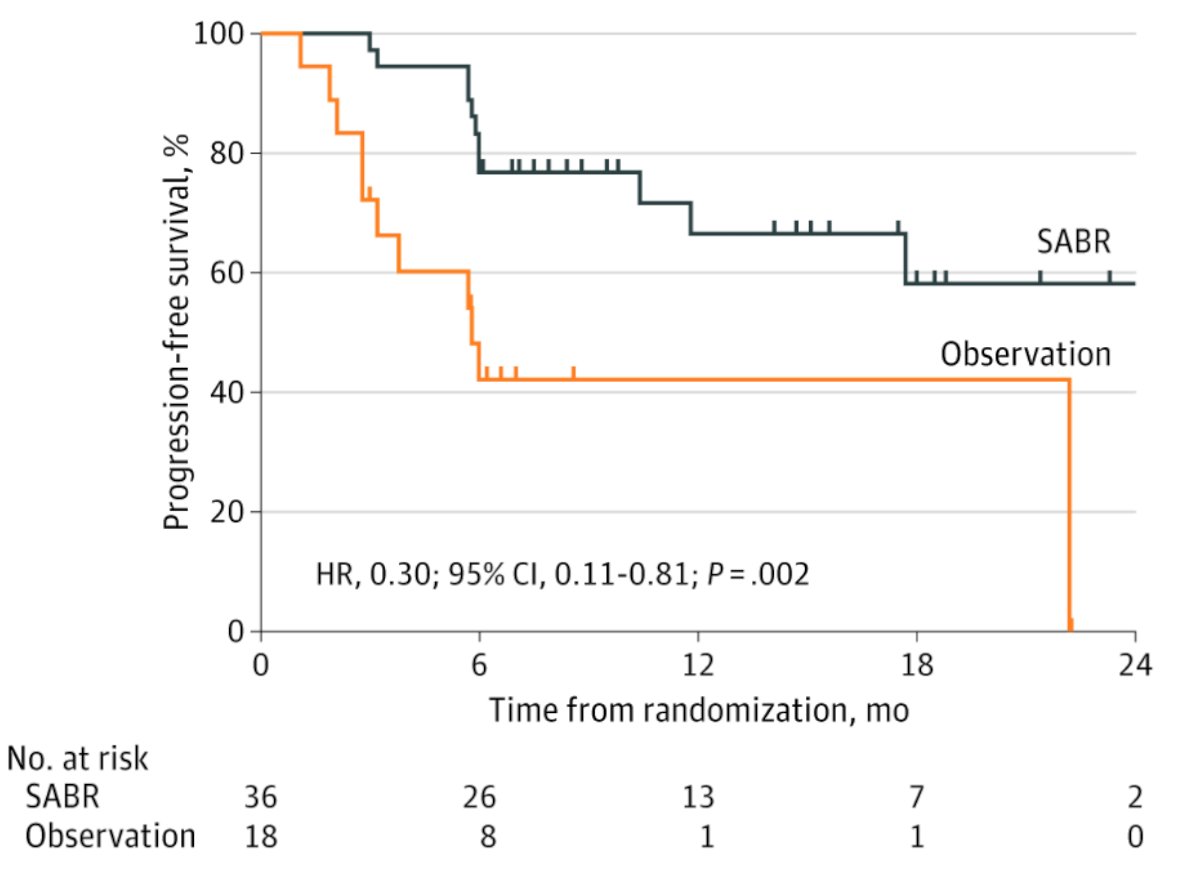
The second phase II trial published recently was the ORIOLE trial,9 randomizing 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):
For those patients in the stereotactic body radiotherapy arm that had a PSMA PET-CT scan, the proportion of men with disease progression at 6 months was 5% in those who did not have any untreated lesions, compared to 38% in those who did have some untreated PSMA avid lesions (p=0.03).
Dr. Mottet concluded his presentation discussion evidence-based treatment options for biochemically relapsed prostate cancer with the following take-home messages:
- A systemic treatment is an over-treatment
- Treating the PSA only is irrelevant
- Treatment must have a clinical impact
Presented by: Nicolas Mottet, MD, PhD, Department of Urology, University Jean Monnet St. Etienne, Saint-Etienne, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71(4):630-642.
- Cornford P, Grummet J, Fanti S, et al. Prostate-specific Membrane Antigen Positron Emission Tomography Scans Before Curative Treatment: Ready for Prime Time? Eur Urol. 2020 Jul 2;S0302-2838(20)30451-6.
- Van Den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol 2019 Jun;75(6):967-987.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med 2017;376(5):417-428.
- Garcia-Albeniz X, Chan JM, Paciorek A, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015 May;51(7):817-824.
- Bravi CA, Fossai N, Gandaglia G, et al. Long-term Outcomes of Salvage Lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: Not as Good as Previously Thought. Eur Urol 2020 Nov;78(5):661-669.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.


