(UroToday.com) In this presentation, Dr. Richard Cathomas presented results from SAKK 08/16, a randomized double-blind placebo-controlled phase II trial of darolutamide maintenance in metastatic castration resistant prostate cancer (mCRPC) previously treated with novel hormonal agents (NHA) and non-progressive disease after subsequent treatment with a taxane. This study sought to assess whether an immediate switch to darolutamide after disease stabilization with taxane chemotherapy for patients with mCRPC and prior NHA treatment might improve radiographic progression-free survival (rPFS).
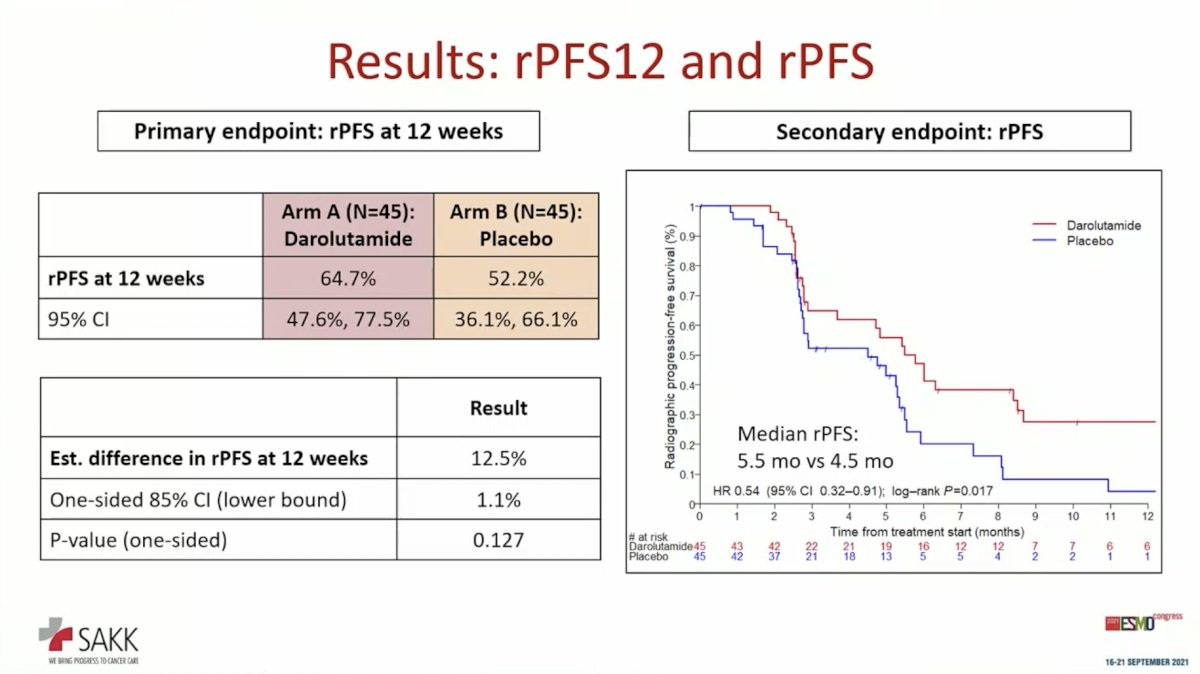
SAKK 08/16 enrolled men previously treated with enzalutamide and/or abiraterone prior to therapy with taxane chemotherapy. Eligible patients with non-progressive disease after taxane chemotherapy (docetaxel or Cabazitaxel) started on trial within 2-8 weeks following last dose of taxane. Participants were randomized to darolutamide until progression versus placebo. The primary endpoint was rPFS at 12 weeks after treatment start (rPFS12).

The study randomized 92 patients, 90 of whom were eligible for analysis. The two arms were well-balanced for demographic and baseline characteristics. Compared to placebo, darolutamide resulted in a significantly rate of rPFS12 (64.7% versus 52.2%; P = 0.125; prespecified threshold for significance P < 0.15). The secondary endpoint of rPFS was also significantly prolonged improved with maintenance darolutamide (5.5 months versus 4.5 months; HR 0.54, 95% CI 0.32-0.91; P = 0.017), though only by one month.
Event-free survival (which includes PSA and clinical progression) was significantly prolonged by the addition of darolutamide (median 5.4 versus 2.9 months; HR 0.46, 95% CI 0.29-0.73; P < 0.001). There was a trend towards improvement in overall survival on the darolutamide arm (24.0 versus 21.3 months; HR 0.62, 95% CI 0.3-1.3; P = 0.18), though this was not statistically significant. The waterfall plot of PSA response clearly favored darolutamide over placebo. A 50% reduction in PSA was observed in 22% of patients treated with darolutamide compared to 4% on the placebo arm.
Subgroup analysis based on response to the latest NHA therapy was informative. Objective radiographic response (complete response or partial response) to prior NHA appeared to be predictive of rPFS and OS benefit to darolutamide maintenance therapy following taxane chemotherapy.
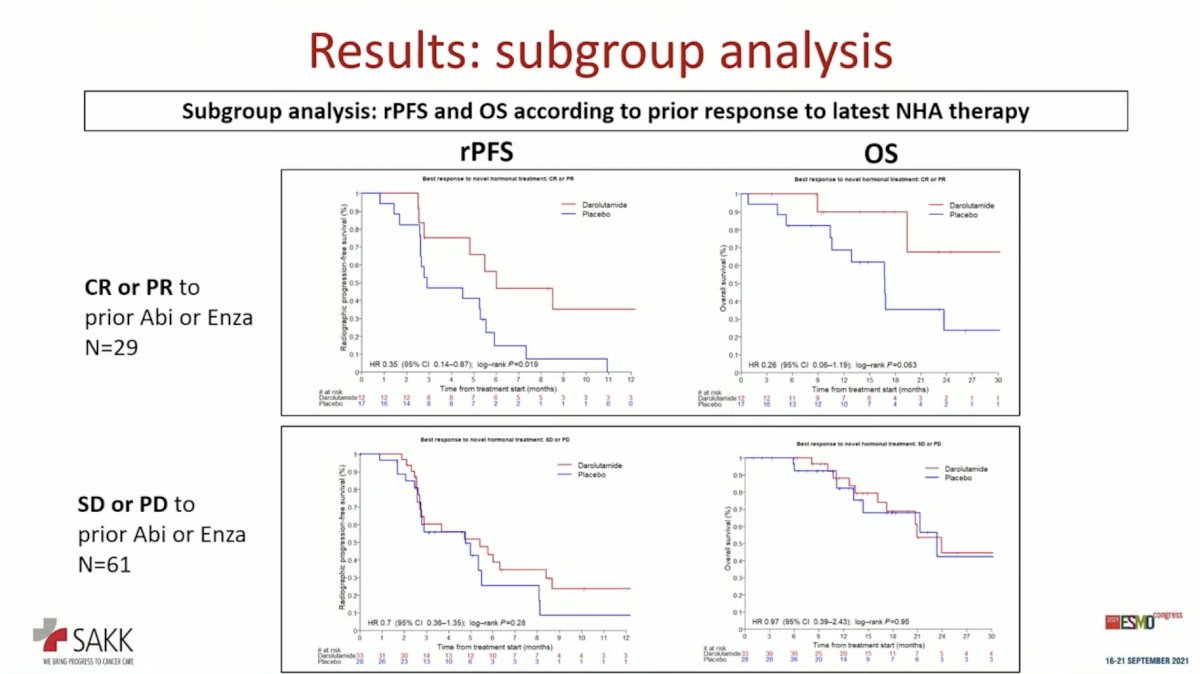
Consistent with its favorable toxicity profile, darolutamide was well-tolerated. Dr. Cathomas concluded that this study met its primary endpoint with a statistically significant, but clinically modest improvement in rPFS. That prior response to NHA appeared to predict benefit from maintenance treatment after NHA and taxane provides hypothesis-generating data for future trial design with this approach.
Presented by: Richard Cathomas, MD, Deputy Head of Oncology/Haematology at the Kantonsspital Graubünden and Associate Professor at the University of Zurich
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist, Dana-Farber Cancer Institute (Twitter: @jberchuck) during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.


