(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 annual meeting’s non-prostate cancer session included a presentation by Dr. David McDermott discussing long-term outcomes in the KEYNOTE-427 Cohort A trial of pembrolizumab monotherapy for first-line treatment of advanced renal cell carcinoma (RCC). A number of combination therapies involving immune checkpoint inhibitors, including the PD-1 inhibitor pembrolizumab, have shown antitumor activity as first-line treatment of advanced RCC. In the open-label, single-arm, phase II KEYNOTE-427 study (NCT02853344), first-line pembrolizumab monotherapy showed antitumor activity in patients with advanced clear cell RCC (cohort A).1 At the 2021 ESMO congress, Dr. McDermott and colleagues presented updated efficacy and safety results after a minimum of 41 months of follow-up for patients with clear cell RCC.
Patients with histologically confirmed clear cell RCC and measurable disease per RECIST v1.1 who had not previously received systemic therapy were given pembrolizumab 200 mg IV every 3 weeks for up to 35 doses or until progressive disease, unacceptable toxicity, or withdrawal of consent. The primary endpoint was ORR per RECIST v1.1 by a blinded independent review. Secondary endpoints included duration of response and PFS per RECIST v1.1 by blinded independent review, OS, and safety. The trial design for KEYNOTE-427 is as follows:
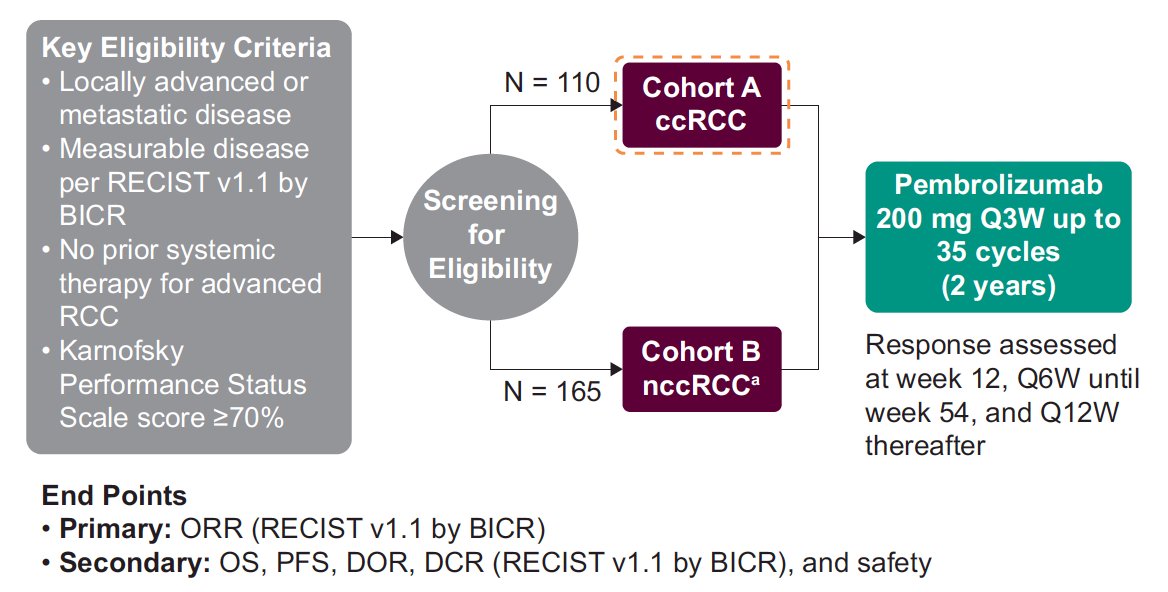
Of 110 enrolled patients, 42 (38.2%) patients had favorable IMDC risk and 68 (61.8%) had intermediate/poor risk. As of February 5, 2021, median duration of therapy was 8.5 months (range, 0.03-26.7), and the median time from enrollment to data cutoff date was 47.3 months (range, 40.9-51.7). ORR was 36.4% (95% CI, 27.4-46.1; 4 [3.6%] complete responses, 36 [32.7%] partial responses), and the median duration of response was 18.9 months (95% CI, 7.1- 37.7); an estimated 43.5% of responders remained in response for ≥24 months. Overall, 69.0% had a reduction in the sum of target lesions, including 19.1% that had an ≥80% reduction in the sum of target lesions. By IMDC risk category, ORR was 31.0% (95% CI, 17.6-47.1) in patients with favorable-risk and 39.7% (95% CI, 28.0-52.3%) in patients with intermediate/poor risk. For all patients, median PFS was 7.1 months (95% CI, 5.6-11.0) and median OS was 40.7 months (95 CI, 31.1 to not reached); 30-mo PFS and OS rates were 19.9% and 62.6%, respectively. In patients with favorable risk, median PFS was 9.7 months (95% CI, 5.6-12.4) and median OS was not reached (95% CI, 40.1 to not reached); PFS and OS rates at 30 months were 16.4% and 78.3%, respectively. In patients with intermediate/poor risk, median PFS was 6.9 months (95% CI, 3.3-11.0) and median OS was 30.8 months (95% CI, 23.8-47.1); PFS and OS rates at 30 months were 22.4% and 52.9%, respectively. As follows are the Kaplan-Meier curves for PFS and OS stratified by IMDC risk category:

No new safety signals were observed.
Dr. McDermott concluded his presentation of the updated results of the KEYNOTE-427 Cohort A patients with the following take-home messages:
- After a minimum of 41 months, pembrolizumab monotherapy continued to show antitumor activity as first-line therapy in patients with clear cell RCC across IMDC risk groups
- The safety and tolerability profile was consistent with the previously reported results for this cohort and the established safety profile of pembrolizumab monotherapy
Presented by: David F. McDermott, MD, Hematology/Oncology, Beth Israel Deaconess Medical Center, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:


