To test this hypothesis, the authors propose a phase 1 lead-in study followed by a randomized phase 2 trial. Patients with Gleason 9 or 10 localized prostate cancer and PSA less than 150 who have not received prior ADT will be eligible for the phase 1 lead-in. The lead-in will consist of escalating doses of the PARP inhibitor niraparib. The schema for the lead-in and anticipated recruitment numbers are shown below.
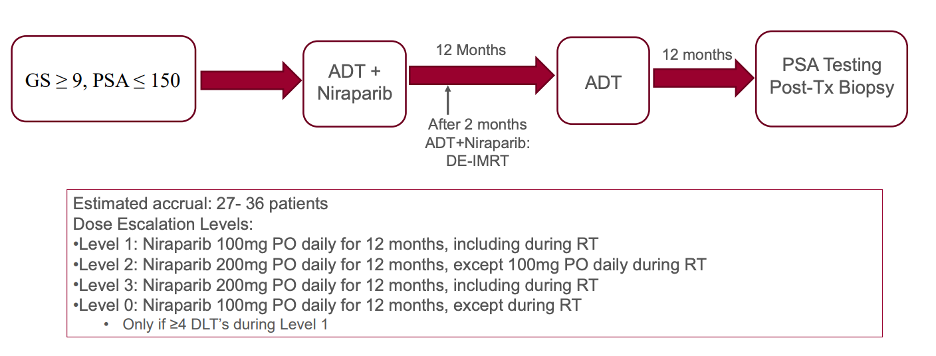
Once the maximum tolerated dose of niraparib is established, the phase 2 study will begin. The schema for the phase 2 randomized trial is shown below.
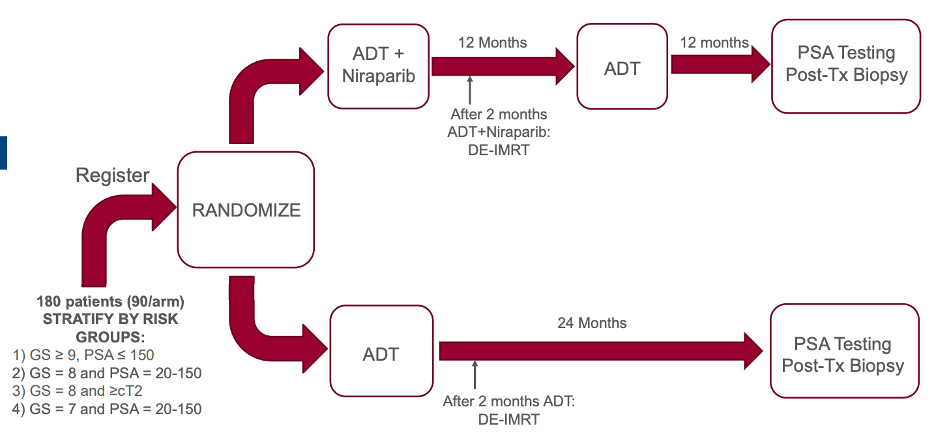
The primary outcome in the phase 2 study is compare the number of patients considered disease free (PSA < 0.1 in this study) between treatment arms. Secondary endpoints will include survival, locoregional progression, rates of development of metastatic disease, and exploratory tissue and blood biomarker studies to identify correlates of resistance or response to therapy.
In both arms, radiation will be delivered in either a standard (79.2Gy in 44 fractions) or hypofractionated (70Gy is 28 fractions) manner along with 45 Gy in 25 fractions to the pelvic lymph nodes. Brachytherapy is not allowed. Testosterone suppression will be accomplished per investigator discretion and will start 2-4 months prior to radiation therapy.
Presented by: Zachary S. Zumsteg, MD, Assistant Professor of Radiation Oncology, Cedars-Sinai Medical Center, Los Angeles, CA
Written by: Alok Tewari, MD, PhD, Medical Oncologist at the Dana-Farber Cancer Institute, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


