In a proffered paper presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Zeynep Zengin, MD, presented the results of a cohort study utilizing a commercially available ctNDA assay.
The authors identified 847 consecutive patients with advanced renal cell carcinoma who underwent ctDNA testing utilizing the commercially available Guardant360 platform, a clinically-validated 73 or 74-gene panel. These patients underwent testing between November 2016 and December 2019. A targeted next-generation sequencing assay of ctDNA assessed sequence alterations, small insertions and deletions, amplifications, and fusions.
Further, among a subset of included patients with available tissue (n=47), the authors compared genetic alterations identified in ctDNA assays and those detected via tissue-based testing using either whole-genome sequencing (Ashion Analytics) or targeted next-generation sequencing (Foundation Medicine).
As mentioned, the authors included 839 of 847 (8 excluded due to suspected urothelial histology) patients of whom 600 were men and 247 were women. At least one genetic alteration was identified in 669 of 929 ctNDA samples (72%). Excluding variants of unknown significance and synonymous variants, the most commonly identified genetic alterations identified in ctDNA analysis were TP53 (37%), VHL (22%), and EGFR (6%).
Notably, the authors identified mutations in DNA damage repair genes in approximately 6% of patients.
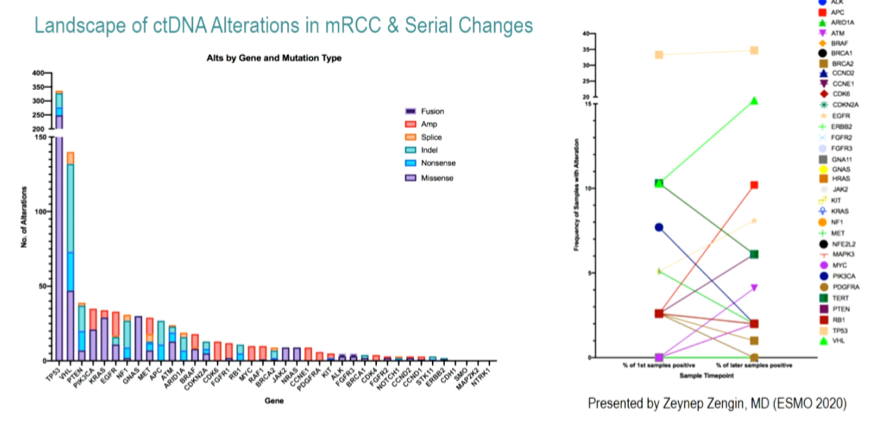
Finally, the authors assessed sequential changes in ctDNA assay results over time among 39 patients. As seen on the right side of the figure above, there were increased mutation rates, particularly in EGFR and PTEN, over time.
Among the 46 patients from whom tissue-based DNA analysis could be performed, the most commonly identified genetic alterations were VHL (64%), PBRM1 (45%), and SETD2 (32%). Notably, PBRM1 and SETD2 were not included in the ctDNA panel. Among those genes included on the ctDNA assay, 154 genetic alterations were found across both assay approaches. Of these 154 alterations, 41 (27%) were identified only in blood samples (red; ctDNA), 92 (60%) were identified only in tissue samples (green), and 21 (14%) were found in both platforms (blue, in the figure below). Notably, the concordance between ctDNA and tissue DNA was 96.2%. Concordance was higher among patients for whom ctDNA and tissue-based testing was performed within 6 months of each other.
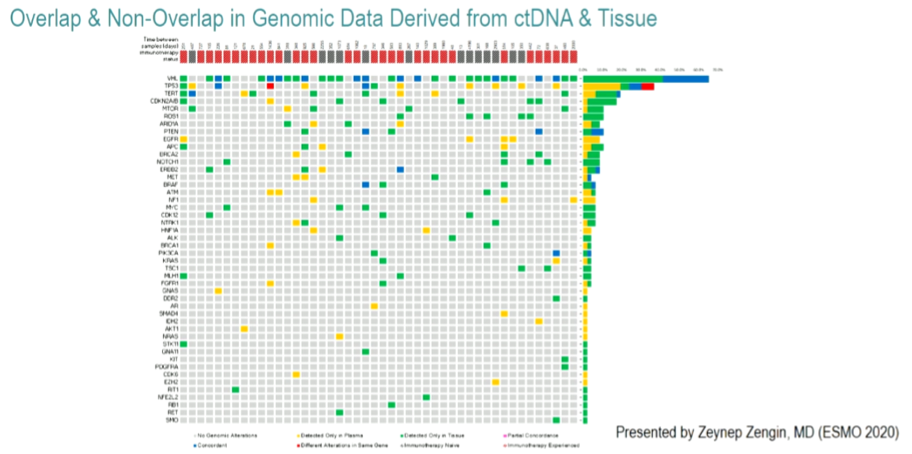
The authors conclude that ctDNA analysis is both feasible in patients with mRCC and highlight concordant with genetic alterations seen on tissue-based analysis. Discrepancies between ctDNA and tissue-based analysis suggest tumor evolution as a result of time and treatment pressures.
Presented by: Zeynep B. Zengin, MD, Postdoctoral fellow, City of Hope, Los Angeles, CA
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Nashville, TN, Twitter: @WallisCJD, at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.


