(UroToday.com) The KEYNOTE-052 trial showed that pembrolizumab monotherapy has durable antitumor activity as first-line therapy in cisplatin-ineligible patients with locally advanced unresectable or metastatic urothelial carcinoma (UC)1. Moreover, pembrolizumab has also shown durable activity as 2nd line therapy in patients with platinum-refractory advanced UC in the KEYNOTE-045 trial2.
Immune gene expression profiling in UC has the potential to identify patients whose tumors are more likely to respond to anti–PD-1/PD-L1 treatment3,4. Several factors have been shown to be associated with resistance to anti–PD-1/PD-L1 treatment in advanced UC5. These include stromal tumor microenvironment, epithelial to mesenchymal transition (EMT), and transforming growth factor-beta (TGF-β) gene expression signatures.
In an exploratory biomarker analysis of KEYNOTE-052, response to pembrolizumab was positively associated with the 18-gene T-cell–inflamed gene expression profile (GEP) and negatively associated with a stromal signature (stroma/EMT/TGF-β)6,7.
The objective of the presented study was to determine the association of T-cell–inflamed GEP and stromal signatures with outcomes in patients treated with pembrolizumab monotherapy in an exploratory analysis of advanced UC patients from the KEYNOTE-045 trial (NCT02256436).
The KEYNOTE-045 trial was an open-label, international, phase 3 trial (N = 542)2 (Figure 1). Patients were randomly assigned 1:1 to receive either pembrolizumab 200 mg every three weeks or the investigator's choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Association of the signatures as continuous variables with the outcomes was assessed in each treatment arm separately, using univariate analysis, performed using logistic regression (objective response rate [ORR]), and Cox proportional hazards (progression-free survival [PFS], overall survival [OS]) modeling, adjusted for Eastern Cooperative Oncology Group (ECOG) performance status.
Figure 1 – KEYNOTE 045 trial: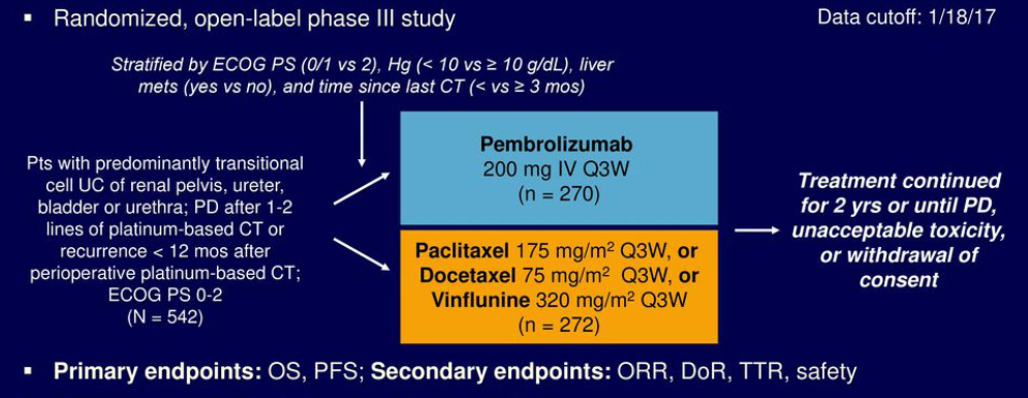
The results demonstrated that 147 of 266 pembrolizumab-treated patients (55%) and 127 of 255 chemotherapy-treated patients (50%) had available RNA-sequencing data. Key baseline characteristics were similar between the overall study population and the population with available RNA-sequencing data.
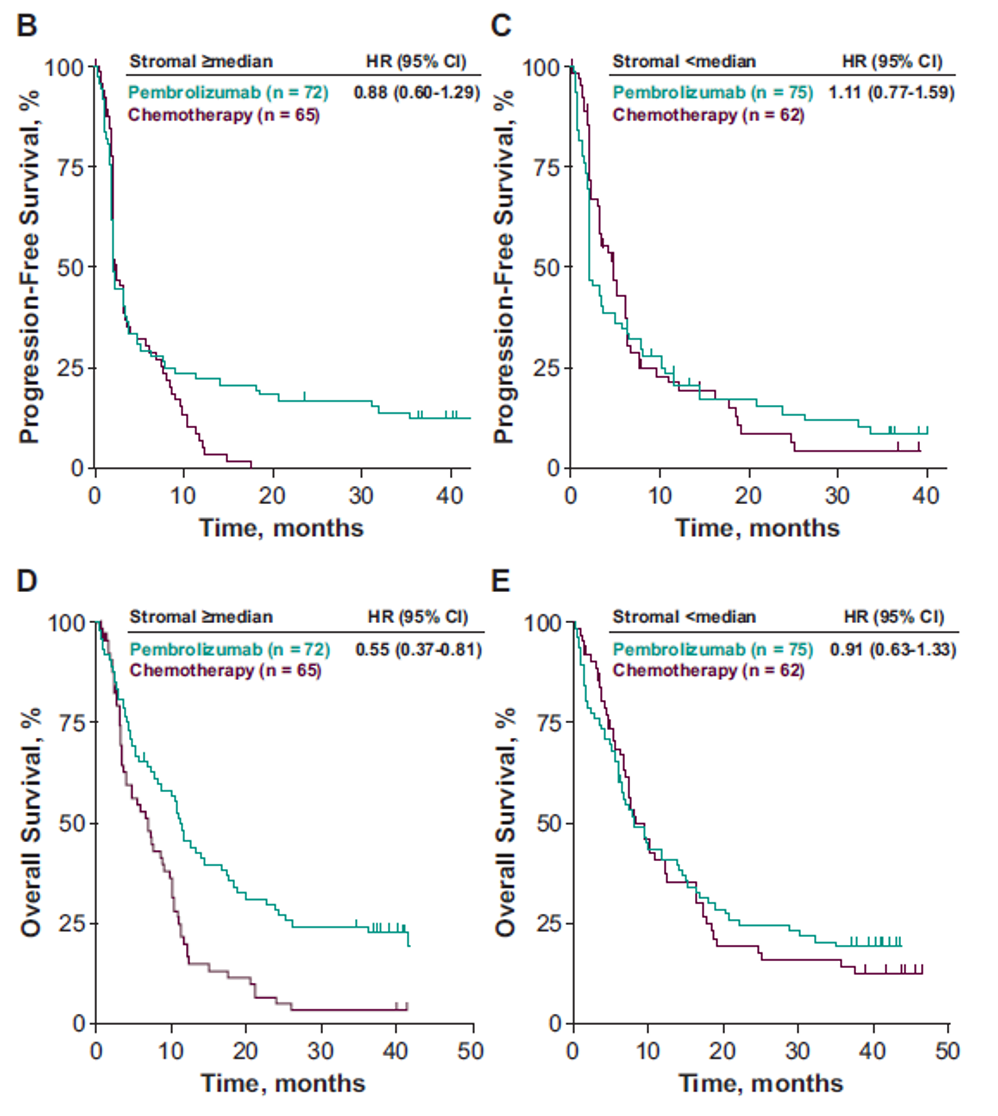
The results show that T-cell–inflamed GEP, as a continuous variable, was positively associated with ORR to pembrolizumab but not to chemotherapy (Table 1). There was no association with PFS or OS in either the pembrolizumab or chemotherapy group (table 1). Pembrolizumab improved PFS and OS vs. chemotherapy in the T-cell–inflamed GEP non-low group (Figure 1). The stromal signature, assessed as a continuous variable, was not associated with favorable outcomes with pembrolizumab (Figures 3 and 4).
Table 1 - Association Between T-Cell–Inflamed GEP/Stromal Signature and Clinical Outcomes in Each Treatment Group: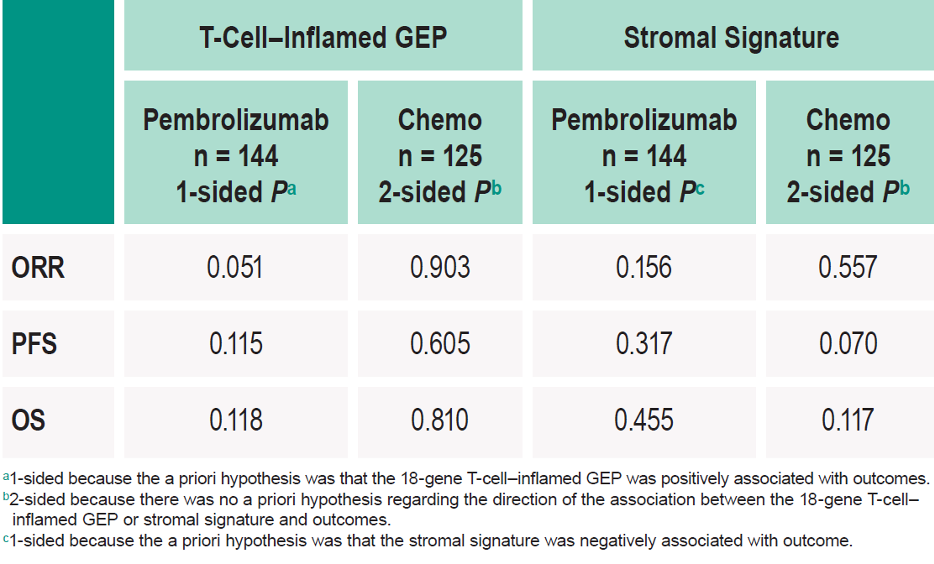
Figure 1- Clinical Utility of the Prespecified T-Cell–Inflamed GEP Cutoff for objective response rate:
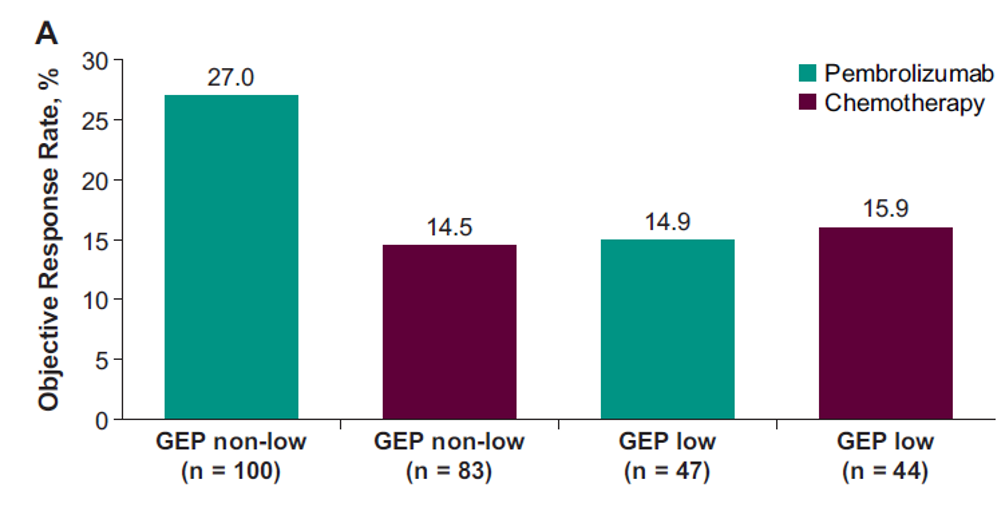
Figure 2 – Clinical Utility of the Prespecified T-Cell–Inflamed GEP Cutoff for PFS and OS:
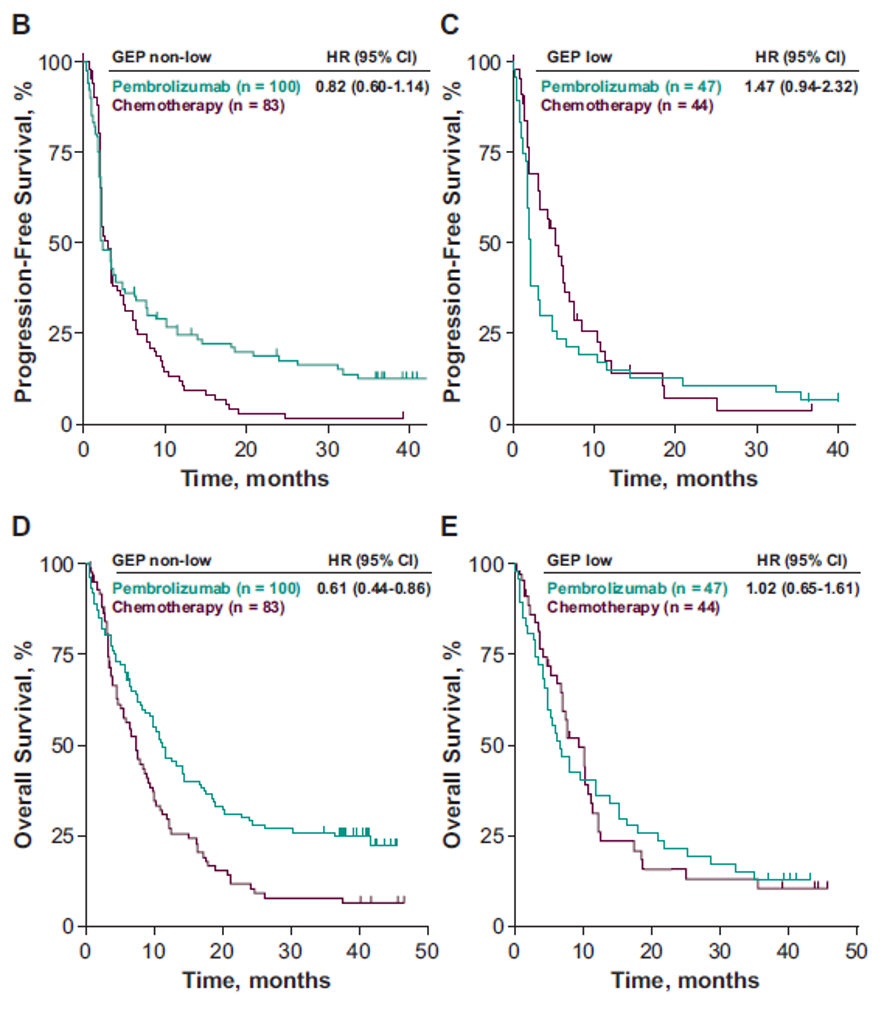
Figure 3 – Clinical Utility of the Prespecified Stromal Signature Median for objective response rate:
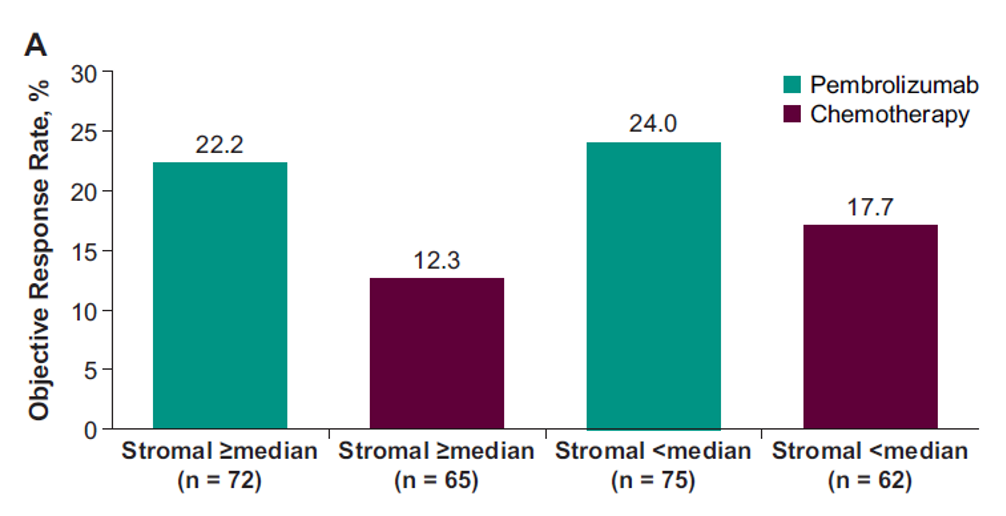
Figure 4 - Clinical Utility of the Prespecified Stromal Signature Median for PFS for stromal >= median, PFS for stromal < median, OS for stromal >=median, OS for stromal <median:
In conclusion, this exploratory biomarker analysis of patients with advanced UC, T-cell–inflamed GEP, as a continuous variable, was positively associated with ORR to pembrolizumab but not chemotherapy. No association with PFS or OS was observed in either the pembrolizumab or chemotherapy group. HR estimates using a prespecified T-cell–inflamed GEP cutoff suggested that pembrolizumab improved PFS and OS vs.
Chemotherapy in the T-cell–inflamed GEP non-low group.
T-cell–inflamed GEP should continue to serve as a useful exploratory gene signature to support the mechanism of action of pembrolizumab in highly inflamed tumors. The stromal signature assessed as a continuous variable was not associated with favorable outcomes with pembrolizumab. HR estimates, using a prespecified stromal median, did not support the hypothesized negative association between increased stromal signature median and response to pembrolizumab previously observed in KEYNOTE-0527.
More analyses are currently exploring differences in the association of the stromal signature with pembrolizumab between KEYNOTE-052 and KEYNOTE-045. It is worth mentioning that patients in KEYNOTE-045 received therapy before enrollment, potentially affecting the tumor microenvironment and limiting the ability of the stromal signature to predict response to pembrolizumab.
Presented by: Dr. Petros Grivas, Associate Professor and the Clinical Director of the Genitourinary Cancers Program at the University of Washington, and an Associate Member of the Clinical Research Division at the Fred Hutchinson Cancer Research Center, Seattle, Washington, United States of America
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
References:
1. Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. The Lancet Oncology 2017; 18(11): 1483-92.
2. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. New England Journal of Medicine 2017; 376(11): 1015-26.
3. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. The Journal of clinical investigation 2017; 127(8): 2930-40.
4. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science (New York, NY) 2018; 362(6411).
5. Wang L, Saci A, Szabo PM, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nature Communications 2018; 9(1): 3503.
6. O'Donnell PH, Plimack ER, Bellmunt J, et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: Results of a phase IB study. Journal of Clinical Oncology 2015; 33(7_suppl): 296-.
7. Grivas P, Castellano DE, O'Donnell PH, et al. Association between stromal/TGF-β/EMT gene expression signature and response to pembrolizumab monotherapy in cisplatin-ineligible patients with locally advanced (unresectable) or metastatic urothelial carcinoma. Journal of Clinical Oncology 2019; 37(7_suppl): 433-.


