Barcelona, Spain (UroToday.com) STAMPEDE previously reported that adding upfront docetaxel improved overall survival (OS) for locally advanced and metastatic patients starting long-term androgen deprivation therapy (ADT).1 At the ESMO 2019 prostate cancer session, Professor Nicholas James presented long-term outcomes for M0 patients using metastatic progression-free survival (mPFS) as primary outcome, previously shown to be a surrogate for OS in M0 patients.2
For this analysis of the STAMPEDE platform, standard of care was ADT +/- radiotherapy to the prostate. There were 460 patients receiving standard of care only, and 230 receiving standard of care + docetaxel (randomized 2:1). Standard survival intention-to-treatment analysis methods used Cox regression models adjusted for all stratification factors, with emphasis on restricted mean survival time for non-proportional hazards. There was 70% power (2-sided α = 0.05) to detect HR = 0.70 for mPFS (new metastases, skeletal related events or prostate cancer death). Secondary outcome measures included failure free survival (FFS) and progression free survival (PFS = mPFS or locoregional progression).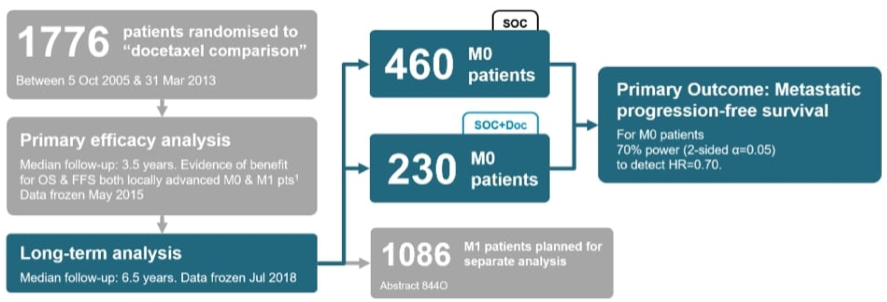
Median follow-up was approximately 6.5 years compared to 3.5 years when last reported, with 142 mPFS events (a 54% increase) on standard of care. Notably, there was no good evidence of an advantage of standard of care + docetaxel over standard of care on mPFS (HR 0.89, 95% CI 0.66-1.19, p = 0.425), with a 5-year mPFS of 82% in standard of care + docetaxel vs. 77% for standard of care alone.
mPFS:
Secondary outcomes showed evidence that standard of care + docetaxel improved FFS (HR = 0.70, 95% CI 0.55-0.88, P = 0.002) and PFS (non-proportional hazard P = 0.033, restricted mean survival time difference = 5.8 months, 95% CI 0.5-11.2, p = 0.031).
FFS: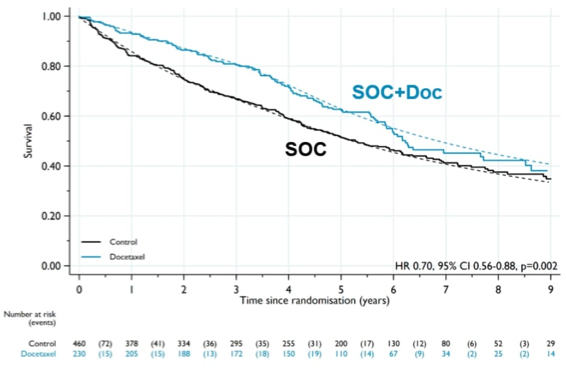
There was no good evidence of a benefit of standard of care + docetaxel on OS (125 standard of care deaths; HR 0.88, 95% CI 0.64-1.21, p = 0.442). There was also no evidence that standard of care + docetaxel increased late toxicity compared to standard of care: after 1 year, grade 3-5 toxicity reported was 29% for standard of care and 30% for standard of care + docetaxel. In a subsequent subgroup analysis, radiotherapy was shown to improve both FFS and PFS in both node-positive and node-negative patients receiving standard of care:
Dr. James concludes this analysis of STAMPEDE in M0 patients with the following conclusions:
- There is robust evidence standard of care + docetaxel improves FFS and PFS
- There is however no good evidence that this translates into a benefit for longer-term outcomes (OS or mPFS)
- There is evidence that radiotherapy improves FFS and PFS in both node-positive and node-negative patients
- The benefits of upfront standard of care + docetaxel for improved FFS and PFS with no excess late toxicity may contribute to treatment discussions
Presented by: Nicholas James, BSc, MB, BS, FRCP, FRCR, PhD, Institute of Cancer and Genomic Sciences NIHR Senior Investigator, Consultant in Clinical Oncology at the Queen Elizabeth Hospital Birmingham and Professor of Clinical Oncology at the University of Birmingham. Birmingham, UK
Co-authors: F. Ingleby,2 N. Clarke,3 C. Amos,2 G. Attard,4 W. Cross,5 D. Dearnaley,6 D. Gilbert,2 R. Jones,7 R. Langley,4 M. Mason,8 D. Matheson,9 C. Parker,10 A. Ritchie,11 H. Rush,2 M. Russell,7 R. Pereira Mestre,12 M. Parmar,2 M. Sydes2
2. MRC Clinical Trials Unit at UCL, London, UK
3. The Christie and Salford Royal Hospitals, Manchester, UK
4. University College London Cancer Institute, London, UK
5. St James University Hospital, Leeds, UK
6. The Institute of Cancer Research (ICR), London, UK
7. University of Glasgow, Glasgow, UK
8. Velindre Cancer Centre Velindre Hospital, Cardiff, UK
9. University of Wolverhampton, Wolverhampton, UK
10. The Institute of Cancer Research/Royal Marsden NHS Foundation Trust, Sutton, UK
11. Gloucestershire Hospitals NHS Foundation Trust, Gloucester, UK
12. IOSI - Ospedale Regionale Bellinzona e Valli, Bellinzona, CH
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain
References:
1. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. 2016;387(10024):1163-1177.
2. Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017 [Epub ahead of print].


