CheckMate 9KD is a Phase II study of nivolumab in combination with docetaxel, rucaparib, or enzalutamide. Eligible patients had confirmed metastatic adenocarcinoma of the prostate, ongoing androgen deprivation therapy, and evaluable tumor biopsy. Patients assigned to nivolumab + docetaxel (chemotherapy-naïve and received ≤2 prior second-generation hormonal therapies) received nivolumab 360 mg every 3 weeks + docetaxel 75 mg/m2 every 3 weeks + prednisone 5 mg twice daily for ≤10 cycles followed by nivolumab 480 mg every 4 weeks alone until disease progression or unacceptable toxicity (up to 2 years). The co-primary endpoints were objective response rate (ORR) and prostate-specific antigen (PSA) response rate (defined as ≥ 50% PSA reduction from baseline). Secondary endpoints included radiographic progression-free survival (rPFS), and safety/tolerability in all treated patients.
The study design for CheckMate 9KD is as follows: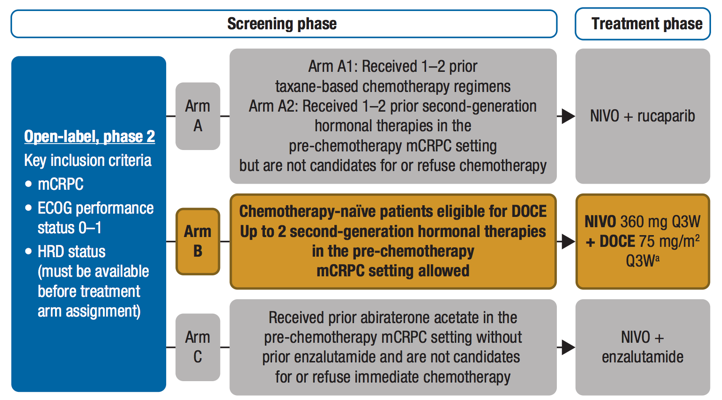
This interim analysis included the first 41 patients in the nivolumab + docetaxel arm with a minimum follow-up of 28 weeks, of whom 19 (46.3%) had measurable disease. The median age of patients was 73 years (range 53-88), all were ECOG 0-1, median PSA was 41 ng/mL (range 1-907), 34.1% had visceral metastases, and 65% had received prior enzalutamide or abiraterone. At the time of the analysis, 24 (58.5%) had discontinued study treatment. The ORR in patients with measurable disease was 36.8% (95% CI 16.3–61.6) with one complete response and six partial responses. Among all 41 treated patients, the confirmed PSA response rate was 46.3% (95% CI 30.7–62.6), the median rPFS was 8.2 months (95% CI 6.6–not estimable), and the 6-month rPFS rate was 71.5%.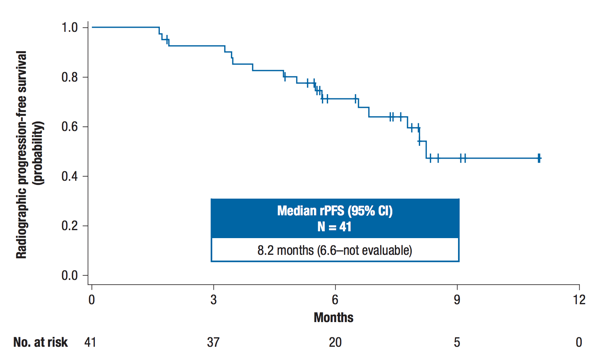
Any-grade adverse events occurred in 92.7% of patients and grade 3/4 treatment-related adverse events occurred in 48.8% of patients. The most common grade 3/4 adverse events were neutropenia (29.3%), febrile neutropenia (9.8%), diarrhea (7.3%), and asthenia (7.3%).
Dr. Fizazi’s conclusions for this initial analysis of the nivolumab + docetaxel arm of CheckMate 9KD were:
- The combination of nivolumab + docetaxel showed encouraging clinical activity in patients with mCRPC, with a confirmed ORR of 36.8% and confirmed PSA response rate of 46.3%
- The safety profile was consistent with those of the individual agents
- A Phase III trial is warranted to further evaluate nivolumab + docetaxel in mCRPC (CheckMate 7DX)
Clinical Trial Identification: NCT03338790.
Presented by: Karim Fizazi, MD, PhD, Professor of Medicine of Institut Gustave Roussy, (IGR) in Villejuif, France
Co-Authors: K. Fizazi1, P. Gonzalez Mella2, D. Castellano3, J.N. Minatta4, A. Rezazadeh Kalebasty5, D. Shaffer6, J.C. Vazquez Limon7, A. Armstrong8, H.M. Sanchez Lopez9, B. Sharkey10, A. Saci11, J. Li10, X. Wang10, M. Ciprotti12, P. Sathyanarayana12, F. Saad13, D. Petrylak14, M. Retz15, R. Pachynski16, C. Drake17
1. Institut Gustave Roussy, Villejuif, FR
2. Instituto Oncológico Vina del Mar, Vina del Mar, CL
3. Hospital Universitario 12 de Octubre, Madrid, ES
4. Hospital Italiano, Buenos Aires, AR
5. Norton Cancer Institute, Louisville, US
6. Albany Medical Center, New York Oncology Hematology, Albany, US
7. Instituto Jalisciense de Cancerología, Guadalajara, MX
8. Duke University, Durham, US
9. Hospital Regional de Alta Especialidad del Bajío, Guanajuato, MX
10. Bristol Myers Squibb, Princeton, US
11. Bristol-Myers Squibb, Lawrenceville, US
12. Bristol-Myers Squibb, Princeton, US
13. Hospital St. Luc du CHUM, Montreal, CA
14. Yale Cancer Center, New Haven, US
15. Technische Universitat München, München, DE
16. Siteman Cancer Center, St. Louis, US
17. Columbia University Medical Center, New York, US
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct, 2019 in Barcelona, Spain
Reference:
- Patel, Amar, and Lawrence Fong. "Immunotherapy for Prostate Cancer: Where Do We Go From Here?—PART 2: Checkpoint Inhibitors, Immunotherapy Combinations, Tumor Microenvironment Modulation, and Cellular Therapies." Prostate 32, no. 6 (2018).


