Dr. Enrique Grande presents the final progression-free survival analysis of IMvigor130 as well as an interim analysis of overall survival. IMvigor130 is a 3-arm randomized placebo-controlled trial of platinum-based chemotherapy alone versus platinum chemotherapy plus atezolizumab versus atezolizumab alone for the treatment of patients with metastatic urothelial carcinoma in the first-line setting. The co-primary endpoints were progression-free and overall survival, and the precise chemotherapy regimen (cisplatin versus carboplatin) was deferred to the clinical judgments of the treating investigators.

A total of 1213 patients were randomized. All 3 arms were generally well-balanced with respect to age (median 67-69) and PD-L1 status. However, it is noteworthy that a preponderance of patients in the combination atezolizumab plus platinum arm were deemed cisplatin-ineligible per Galsky criteria and a slightly higher proportion of these patients went on to receive carboplatin (70%) relative the platinum chemotherapy plus placebo arm (66%).

The study met its primary endpoint, with atezolizumab plus platinum chemotherapy achieving a statistically significant improvement in progression-free survival (hazard ratio 0.82, p(one-sided) = 0.007). The chemo-immunotherapy combination was associated with a 1.9-month improvement (8.2 months versus 6.3 months) in median progression-free survival.

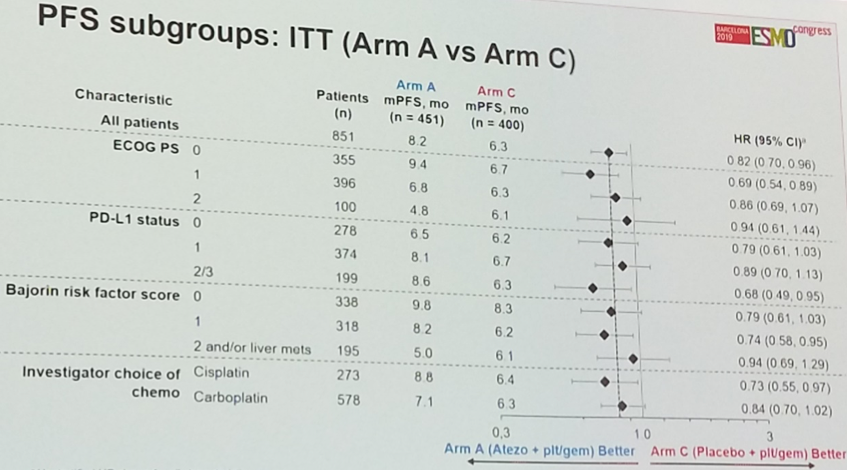
Subgroup analyses generally favored atezolizumab plus platinum-based chemotherapy. Interestingly, the IC2/3 (PD-L1-high) subgroup and the subgroup treated with cisplatin both exhibited a statistically significant improvement in progression-free survival in favor of the combination.
In terms of overall survival, while there was a trend toward improved survival with the combination, this result did not reach statistical significance (HR 0.88 95%CI [0.69 – 1.00]). Nevertheless, atezolizumab plus platinum chemotherapy was associated with a numerically higher median overall survival (16.0 months) compared to platinum chemotherapy plus placebo (13.4 months).

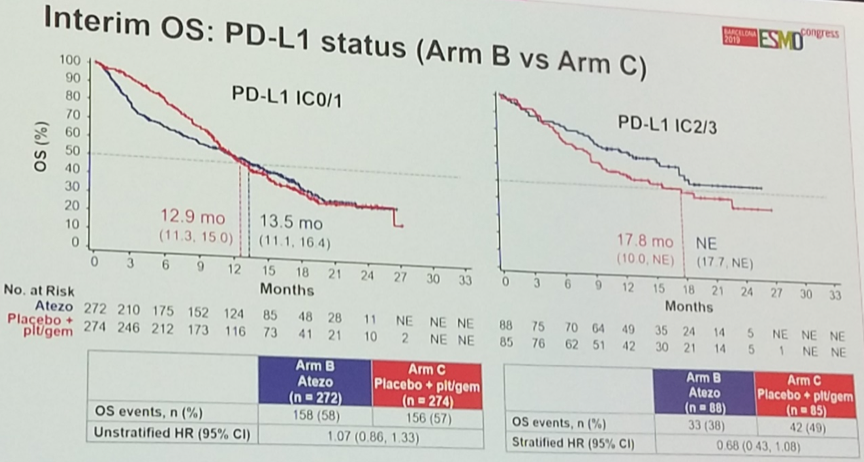
Per protocol, results were stratified according to PD-L1 status. While no significant difference was observed with respect to overall survival among PD-L1-low patients, there was a trend – though not statistically significant – toward benefit with combination chemo-immunotherapy in the PD-L1-high subgroup, suggesting that the use of PD-L1 as a predictive biomarker might be useful in this clinical setting.
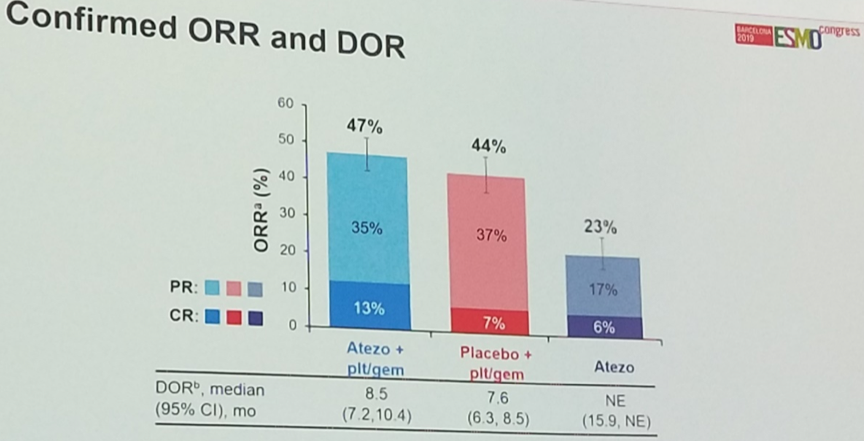
Objective response rates were similar between the arms, though the combination arm did achieve a higher rate of complete responses (13%) relative to the platinum plus placebo arm (7%).
With respect to the safety of the combination, the addition of atezolizumab does not appear to grossly alter the overall safety profile of the regimen, and the most common treatment-related adverse events were similar between the arms. A total of 34% of patients in both arms discontinued treatment for toxicity. Grade 5 events occurred in 9 patients (2%) in the combination arm and 4 patients (1%) in the chemotherapy plus placebo arm.
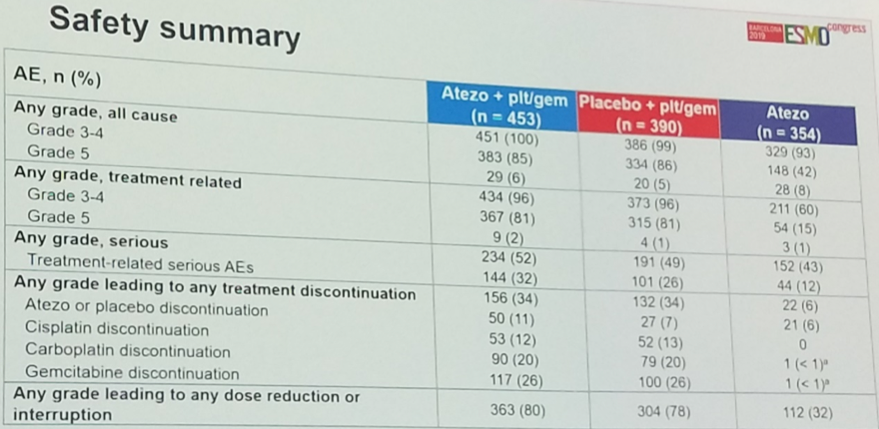
The most common immune-related adverse events were rash, hepatitis, and thyroid abnormalities, all of which skewed toward the chemo-immunotherapy combination arm. No new safety signal was identified.

IMvigor130 is the first positive randomized trial of chemo-immunotherapy in metastatic urothelial cancer. The combination of platinum-based chemotherapy with the immune checkpoint inhibitor atezolizumab is associated with an improvement in progression-free survival that is statistically significant. Overall survival data are not yet mature, though these data have the potential to become practice-changing in the future should the treatment arms continue to diverge in subsequent follow-up analyses.
Presented by: Enrique Grande, MD, MD Anderson Cancer Center, Madrid, Spain
Written by: Michael Lattanzi, MD, Medical Oncology Fellow, Memorial Sloan Kettering Cancer Center, Twitter: @MikeLattanzi at the 2019 European Society for Medical Oncology Congress (#ESMO19), September 27 – October 1, 2019, Barcelona, Spain
References:
1. Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016 May 7;387(10031):1909-20.
2. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017 Jan 7;389(10064):67-76.


