The background for this study is that men with metastatic prostate cancer are treated typically with systemic therapy. Until 2013, the standard of care had been ADT (androgen deprivation therapy) alone. In the past few years, with the reporting of the CHAARTED, LATITUDE and STAMPEDE-abiraterone trials, the new standard for metastatic hormone naïve prostate cancer is ADT and either docetaxel or abiraterone acetate.
Yet, the authors hypothesized that treatment to the primary tumor would provide an overall survival benefit to men with mHNPC, and that the survival benefit would be greatest in men with a lower metastatic burden.
The basic study design was a 1:1 randomized trial comparing ADT (+/- docetaxel at the physician’s discretion) vs. ADT + prostate radiotherapy (+/- docetaxel at the physician’s discretion). The docetaxel addition was based on CHAARTED data. The radiotherapy was given as prostate only radiation (not whole pelvis) and could be given as 2 possible regimens:
- 36 Gy/6 fractions/6 weeks or 55Gy/20 fractions/4 weeks (weekly vs. daily)
In terms of outcomes, the primary outcome was overall survival. The secondary outcomes being reported today include failure-free survival, symptomatic local events, and toxicity. Other secondary outcomes are reported in the manuscript.
Initial target was 1250 men. However, this was revised to 1800, and despite this, they still overaccrued. Of note, CHAARTED was reported after this study had already started accruing – so the study was modified after initiation to account for a metastatic burden. This is seen below:

Baseline characteristics amongst the two groups were equally balanced. Median age 68. Median PSA 97-98. 42% low metastatic burden, 57-58% high metastatic burden. 18% had prior docetaxel use in both arms.
He then delved into the outcomes of the study.
1) Failure-free survival:
- HR 0.76 favoring RT, p < 0.001
- 3-year 8% survival benefit in the RT arm
2) Overall survival:
- HR 0.92 favoring RT, but p = 0.266 – not clinically significant
- 3-year 3% survival benefit
- Looking at the KM curves, there is no real separation between the curves
3) When looking at Radiotherapy schedule, there was no difference based on RT schedule (p = 0.27)
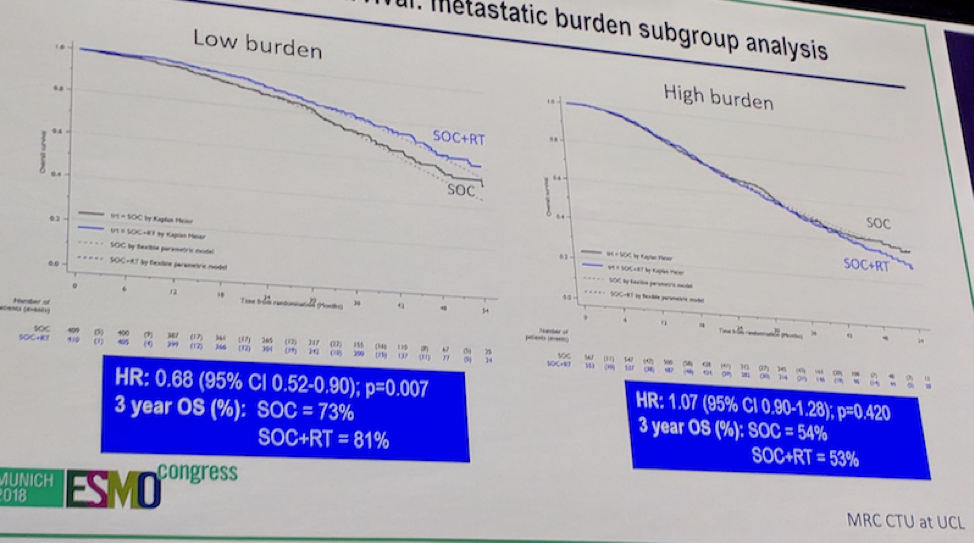
4) Outcomes-based on metastatic burden – this is where the results get more interesting.
- There was clear evidence that response differed by disease burden
- For patients with low volume disease: OS favored RT group (HR 0.68, 95CI 0.52-0.90), p = 0.007
This results in a 3-year OS benefit of 8%
- For patients with high volume disease: OS favored no RT, but CI crossed 1.0 – p = 0.42
In terms of other outcomes:
1) There was no significant difference in time from randomization to second life-prolonging treatment
2) There was no significant difference in time from randomization to first symptomatic local event
- There were more patients in the RT group reporting UTI and catheter use (potentially related to RT related local effects)
- However, there were signs of lower ureteral stent, colostomy and nephrostomy tubes in the RT arm
- Perhaps this data has not yet matured
The key outcome slide is this one: Overall survival by metastatic disease burden
His concluding remarks:
1) Prostate radiotherapy did not improve survival for unselected patients
2) Prostate radiotherapy DID improve survival for patients with low-metastatic burden HNPC (3-year 8% OS benefit, HR 0.68, p = 0.007)
3) Prostate radiotherapy was well tolerated
Based on this, he feels prostate radiotherapy should be standard of care in men with low metastatic burden in addition to systemic therapy. He also feels that this may be translatable to patients with oligometastatic N1 disease.
Invited Discussant:
Dr. Bristow, who is a recent transplant from the University of Toronto to the University of Manchester, was not directly involved in the study.
He began by discussing the concept of oligometastatic disease. Oligometastatic disease represents patients on the lower end of the metastatic spectrum. While not easily defined, there are some features implicit in this terminology:
- A less aggressive disease course
- A unique subset of patients may benefit from a more aggressive local therapy treatment course
- Unique molecular or clinical characteristics that may benefit from a multimodality approach
Therefore, the natural question is whether surgery or ablative therapies to the primary may provide benefit?
After a brief review of the data presented by Dr. Parker, Dr. Bristow addressed some other issues related to this topic.
1) Role of alternative systemic therapies – it is not yet certain if the addition of docetaxel alone (which was not assessed specifically in this study) was of benefit. More importantly, as seen in the earlier talks today, the SOC is likely no longer ADT alone, but rather ADT + abiraterone or ADT + docetaxel. The role of RT needs to be assessed in this setting.
- The PEACE-1 trial is an ongoing trial that will help address this – almost done accruing.
2) The STOPCAP M1 collaborators, led by the Manchester group, in collaboration with HORRAD and STAMPEDE trial groups, is producing the first systematic review and meta-analyses of the two studies in this disease space. The manuscript has been submitted and will be published soon.
3) Role of RT versus surgery in this setting. There are numerous ongoing clinical trials looking a the role of surgery in patients with low-volume metastatic disease, including the g-RAMMP and TROMBONE studies. If positive, these trials may begin to inform the need to evaluate surgery vs. radiation directly.
4) Radiotherapy – prostate alone versus whole pelvis is the other main question here. Particularly in the setting of node-positive disease, is there value to WP radiation? The current study only allowed for prostate radiation alone.
- Longer term follow-up from RTOG studies will help inform re: toxicity of WP vs. prostate only radiation
5) What are the implications for MDT (metastasis direct therapy)? Multiple retrospective studies have demonstrated a benefit to MDT. STOMP (R-phase II) is a clinical trial has started to validate this concept, albeit in a small population of 62 patients. Median ADT-free survival was improved by 8 months with the addition of MDT.
- There are 2 ongoing Phase III clinical trials (CORE and PCX IX) that will help inform us regarding this management strategy
Following this extended discussion, his take-home points were as follows:
1) This is an excellent practice-changing clinical trial that provides a new PREDICTIVE assay (low-volume disease) for local RT in mHNPC
2) RT to prostate should be added to standard of care systemic therapy (either ADT+abi or ADT alone) for newly diagnosed low-volume mHNPC
- The results may be extrapolated to N+ disease
3) Surgical trials, ongoing, will help determine the role of surgery as a local therapy
4) Future trials will help address the role of RT volume (prostate only vs. WP radiation), surgery vs. radiation, modern imaging, systemic therapies and MDT
Presented by: Chris C. Parker, MD, Institute of Cancer Research, Sutton, Great Britain
References:
CHAARTED: Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
STAMPEDE: James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
LATITUDE: Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, twitter: @tchandra_uromd, @TjuUrology at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


