Munich, Germany (UroToday.com) LATITUDE is a double-blind, placebo-controlled, phase 3 trial which randomized patients with metastatic castration sensitive prostate cancer (mCSPC) to ADT + Abiraterone + Prednisone or ADT + Placebo1. In addition to having metastatic disease, eligibility also required the presence of two of three high-risk features, including a Gleason score of 8, at least 3 bone lesions, or the presence of visceral metastases.
This landmark study demonstrated that there is an overall survival benefit for giving upfront abiraterone in patients with newly diagnosed metastatic prostate cancer, similar to the results seen in STAMPEDE2. In LATITUDE, median overall survival was not reached compared with 34.7 months in the placebo group, with a hazard ratio for death of 0.62. In STAMPEDE, the hazard ratio was 0.61. These two studies led to the approval up front abiraterone and were practice changing. This abstract explores the relationship between PSA response and correlation with overall survival and radiographic progression-free survival.
597 patients received abiraterone and 602 patients received placebo in the LATITUDE study and confirmed PSA responses were assessed based on PCWG2 criteria3. The median baseline PSA was 23.85 ng/mL. In this abstract, patients who achieved a 50% and 90% decrease in PSA were compared with patients who did not respond to abiraterone (PSA50 and PSA90). Patients who achieved a PSA50 had a HR for death of 0.435 and patients who had a PSA90 had a HR for death of 0.107 – thus, abiraterone reduced the risk of death by 56% if you had a PSA50 and 89% if you achieved a PSA90. 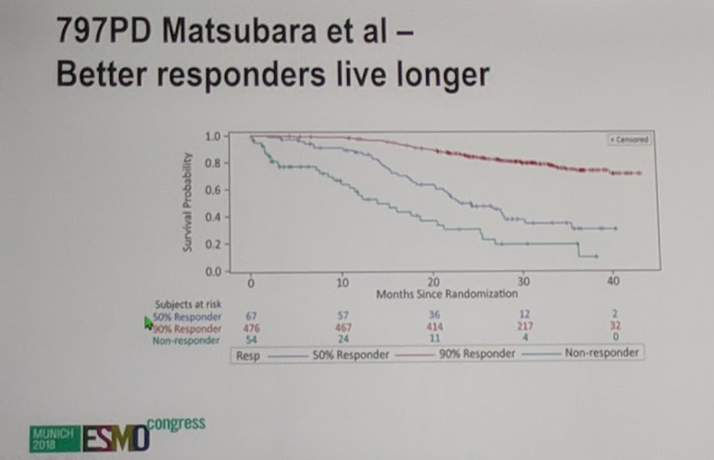
Compared to placebo + ADT, AAP increased the percentage of patients achieving a PSA <0.2 at 3, 6, and 12 months. 58% of patients receiving AAP attained a PSA<0.2 compared with 12.8% of patients on placebo+ADT. The median PSA nadir for patients on AAP was 0.09 ng/mL compared with 2.36 for patients on placebo+ADT. The time to nadir was explored as well and those who have a greater time to nadir do better, as they typically have a lower nadir, which is better for prognosis. AAP dramatically increased the time to PSA progression compared with placebo+ADT (33.2 months vs 7.4 months), and this time to PSA progression strongly correlated with radiographic PFS.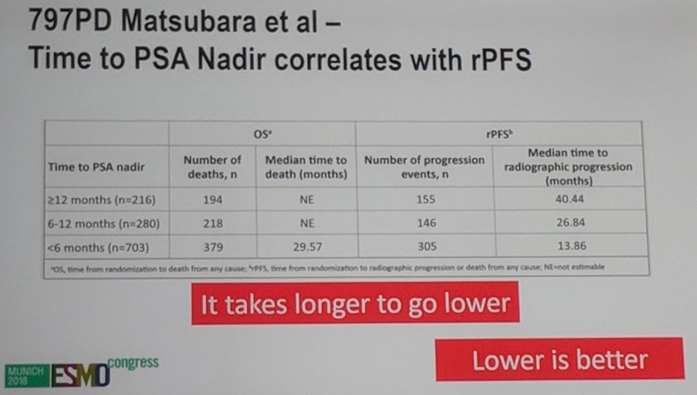
Abiraterone and docetaxel have greatly improved the overall survival of our patients with metastatic castration sensitive prostate cancer (mCSPC). This is terrific for our patients but has made it more challenging to design clinical trials as median overall survival data has not yet been reported for LATITUDE but is over 3 years and radiographic progression-free survival was 30.8 months. Thus, several investigators are looking to discover surrogate endpoints which may help guide future clinical trial development.
The discussant raised several important points regarding the data variables discussed here, namely PSA nadir and time to PSA nadir. While these variables were prognostic, they can only be determined in a retrospective fashion and thus are challenging to use in the clinic. In terms of PSA response and correlation to rPFS and OS, this is clearly dependent on the therapy and thus will not always correlate with overall survival. This will be very challenging to use as PSA response clearly depends on the therapy, and there have been some therapies with minimal PSA response but with overall survival, namely Radium 2234 and Sipeulecel-T5.
During the Q&A session, several additional points of analysis were brought up which may be interesting to correlate with overall survival, including the rate of PSA decline and early PSA response at 4 weeks. It will also be important to establish relationships between the biology of patients and their therapies with PSA and radiographic response.
Presented by: Nubaki Matsubara, Kashiwa, Chiba, Japan
References:
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. New England Journal of Medicine 2017;377:352-60.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. New England Journal of Medicine 2017;377:338-51.
3. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2008;26:1148.
4. Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. New England Journal of Medicine 2013;369:213-23.
5. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New England Journal of Medicine 2010;363:411-22.
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


