As part of this presentation, the authors specifically assess the role of PD-L1 status as a predictor or prognostic marker of clinical outcomes in patients treated in these two trials. Why is this relevant? Because the ipilimumab/nivolumab combination was simultaneously reported to have a 3-month median PFS benefit in the 1st line for patients with advanced intermediate/poor risk mRCC (CheckMate-214, Motzer RJ NEJM 2018). As these are immune checkpoint inhibitors, PD-L1 status may help determine which patients should be treated with Ipi/Nivo vs. cabozantinib (CAB).
The authors utilized a dual-immunohistochemistry (dual IHC) staining for PD-L1 in both the tumor and immune cells (formalin-fixed paraffin-embedded baseline tumor tissue, pre-treatment). This included 110 patients from CABOSUN and 306 patients from METEOR. They then used novel digital image analysis algorithms that allowed scoring of PD-L1 staining on each set of cells. First, they used Fisher’s exact test to compare RECIST overall response rate (ORR) between PD-L1 positive (>=1% cutoff) vs negative tumors. They then correlated this score to PFS and OS using Cox-regression analysis.
PD-L1+ staining was found in 29% and 23% of METEOR and CABOSUN patents, respectively.
In univariable analysis, patients who stained positive (PD-L1+ TC) had poorer PFS and OS on both trials independent of therapy.
In the METEOR trial,
-PFS: median 7.2 vs 5.3 mo, p=0.027
-OS: 21.3 vs 15.1 mo, p=0.003
In the CABOSUN trial:
-PFS 8.3 vs 5.5 mo, p=0.05
-OS 28.1 vs 20.8 mo, p=0.05.
On multivariable analysis, however, differences were not statistically significant when adjusted for IMDC prognostic risk groups and bone metastasis.
When looking at specific treatment arms, the use of CAB was associated with improved PFS, OS, and ORR compared to everolimus (METEOR) and sunitinib (CABOSUN) irrespective of PD-L1 expression, which is consistent with the original studies. Results were consistent across PD-L1 measures including IC PD-L1, combined PD-L1 score and using different PD-L1 cutoffs.
Treatment comparison on PFS by PD-L1 expression:
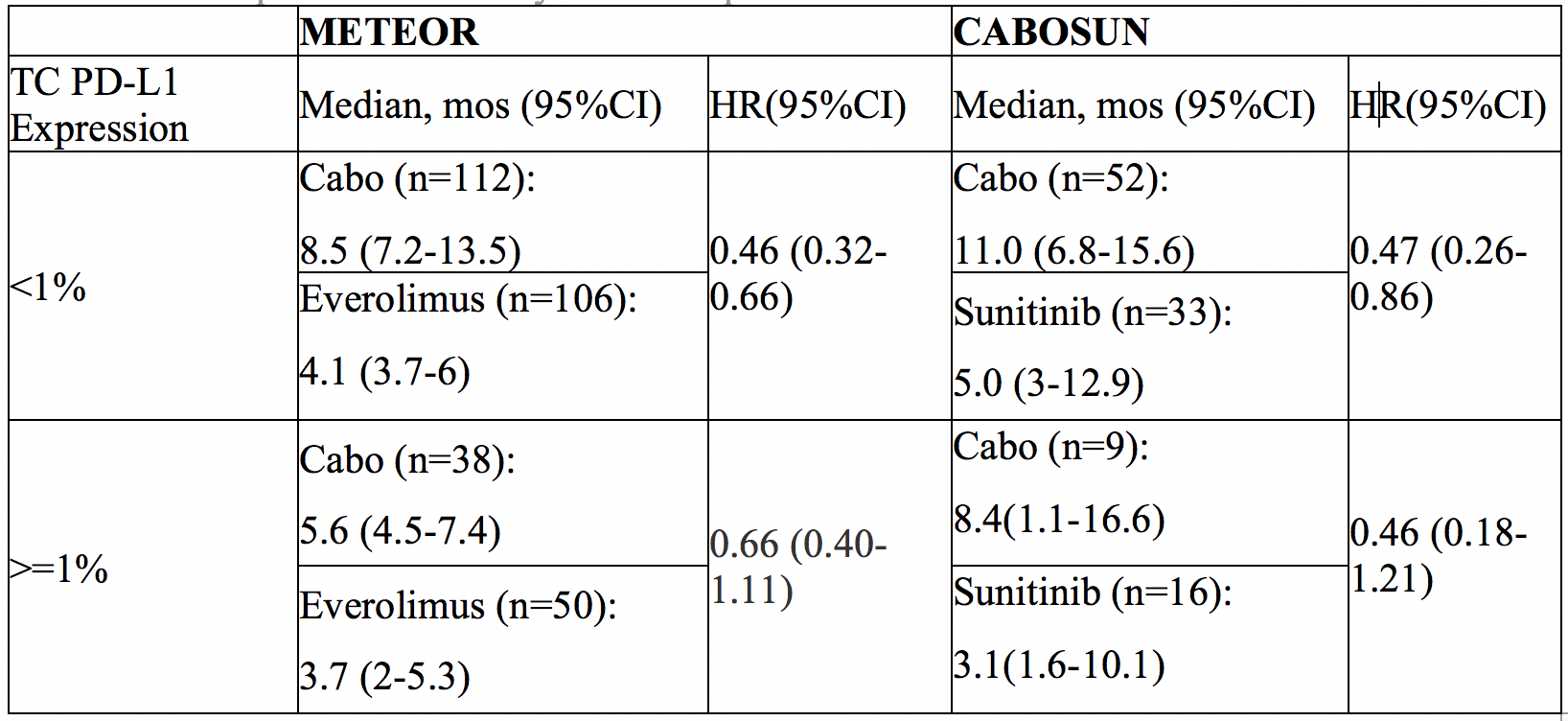
However, the authors don’t specifically compare the use of CAB in PD-L1(-) vs PD-L1(+) patients. While there appears to be a trend towards benefit in PD-L1(-) patients, it does not appear to be significant.
Based on this, the authors conclude that PD-L1 status should not be used to deny patients CAB – when the alternative is everolimus or sunitinib. However, PD-L1 status cannot be used to say that patients should or shouldn’t receive CAB when the alternative is nivo/ipi.
Presented by: Toni K. Choueiri, MD, Dana-Farber Cancer Institute, Department of Medical Oncology, Boston, Massachusetts
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, twitter: @tchandra_uromd, @TjuUrology at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
Further Related Content:
Watch: CABOSUN Trial Plus an In-depth Discussion of Kidney Cancer Treatments - Toni Choueiri


