Three randomized controlled trials (RCTs) have been published to date with conflicting results. Each has had its share of criticisms, as the discussant (see separate report) reviews. The 3 trials in this disease space are the ASSURE (Haas NB et al. Lancet 2016), S-TRAC (Ravaud A et al. NEJM 2016) and PROTECT trials (Motzer RJ et al. JCO 2017) – only S-TRAC was positive for DFS benefit. Based on this, sunitinib has been FDA approved for adjuvant therapy, though uptake has been minimal.
In this latest study, the authors assess the utility of another targeted therapy in this disease space. Axinitib is a selective VEGF 1-3 receptor inhibitor and has been approved for mRCC treatment in the 2nd line. In this ATLAS trial, the authors report the efficacy and safety of axinitib in the adjuvant setting.
The study design is below:
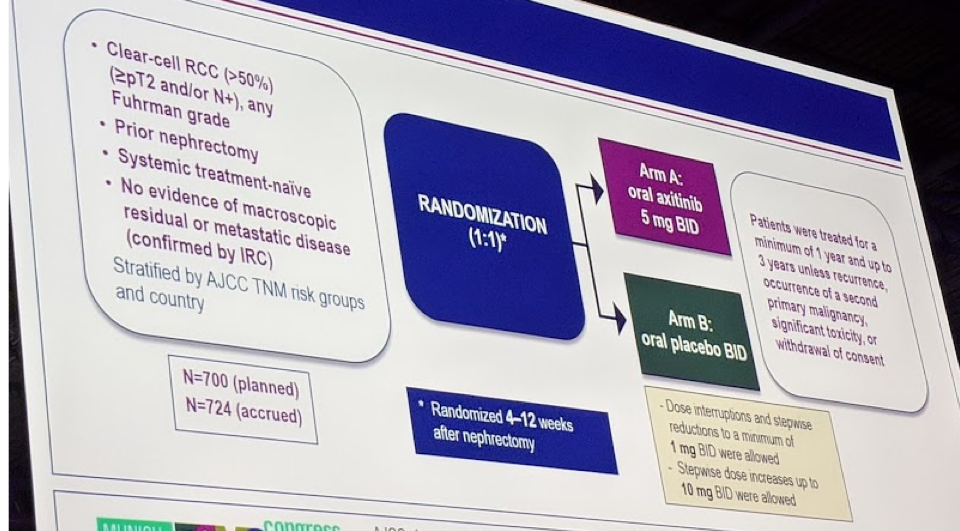
In this study, only patients with clear cell RCC were included. Any patients with >= pT2 or N+ localized RCC and prior nephrectomy were eligible. They had to have no evidence of metastatic disease or residual primary disease. They were stratified later by risk groups, so it was not automatically limited to “high-risk” patients.
Patients were then randomized to axitinib 5 mg twice daily for 1-3 years (1 year minimal, 3 years maximum) or placebo. Dose reduction and increases were allowed incrementally to 1 mg bid or 10 mg bid.
They had a planned accrual of 700 patients, and they met that cutoff – 724 patients were recruited in Asia and Europe.
The primary objective was to demonstrate an improvement in disease-free survival (DFS) in patients who were pT2-4N0Mx or pN1M0. Secondary endpoint was overall survival (OS) and safety/toxicity. They had a planned interim analysis at 203 events and the planned futility stopping boundary was HR 0.81. They did have prespecified subset analyses, which are discussed below – including a separation into “high-risk” (pT3 with Fuhrman grade 3-4 or pT4 and/or pN+ with any T-stage or FG) or “low-risk” (remaining patients).
Over a 3.5 year period, they randomized 724 patients – 363 in AXT arm and 361 in placebo arm. The groups were evenly matched.
- Median age 58
- 69-71% were male
- Interestingly, 73-74% were Asian – only 25% were Caucasian
- 55-57% were “high-risk” while <50% were low-risk
- All patients were clear cell RCC
As expected, the adverse events with AXT were similar to the metastatic setting – no additional AEs noted.
Getting to the crux of the trial – the intent-to-treat DFS was not significantly different between the groups (HR 0.87, p = 0.32).
- In the subgroup analysis of low-risk population – no difference in the investigator-initiated or independent review committee analysis
- In a subgroup analysis of “high-risk” population:
- Investigator-assessed DFS demonstrated a benefit in the AXT group (HR 0.64, p= 0.005)
- However, on independent review, this was not significant – HR 0.735, p = 0.07
- There was a trend towards benefit
- The KM curves do separate (below)

In terms of OS, the curves never separate – there is no signal to suggest benefit. There was no significant difference in OS outcomes.
Ultimately, this was another negative study of adjuvant targeted therapy in the setting of localized RCC. While there may be a signal of benefit in a higher-risk population, we are still not very good at defining this population.
Marine Gross-Goupil, MD, did put up a nice slide to suggest what was different about the ATLAS trial compared to the prior 3 studies – that may account for why it was negative.

Presented by: Marine Gross-Goupil, MD, Bordeaux, France
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @TjuUrology) at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


