With the recent introduction of immune checkpoint inhibitors / immune-oncology agents (ICIs), there has been a natural interest in their role as a neoadjuvant therapy. In this phase 1b/2 study, the authors assess the safety and tolerability of neoadjuvant pembrolizumab in conjunction with chemotherapy for locally advance MIBC – in an effort to see if they can improve upon the historical outcomes.
Eligible patients were: cT2-4aN0M0 urothelial carcinoma or mixed histology.
This was a 2-phase study – one phase looked at patients who were cisplatin-ineligible and the other looked at cisplatin eligible patients. In the second group, which is the only for which they are reporting results today, patients received cisplatin, gemcitabine, and pembrolizumab in a neoadjuvant setting and then underwent cystectomy. Patients received 200 mg IV q3 weeks pembrolizumab x 5 cycles, cisplatin (70mg/m2) day 1, and Gemcitabine (1000mg/m2) days 1 and 8 of a 21 day cycle, for 4 cycles. This was followed by radical cystectomy with node dissection (RC).
They had a total of 40 patients. Basic demographics are as follows: median age 65 yrs, 75% male, 10% had mixed UC histology, and PD-L1 combined positive score ≥10 was 52%.
In terms of tolerability, the first 12 patients were on the phase 1b component. In these patients, there was no drug-limiting toxicity in 6 patients. 3 patients chose not to have cystectomy. The other 3 had Grade 3-5 adverse events. In the entire cohort, Gr 3/4 cytopenias occurred in 57% of pts. Overall, as expected in terms of adverse events.
In terms of drug received, the median number of doses given for pembro=5, C=4, G=8.
Of the 40 patients, only 35 pts who had RC - 4 refused and 1 had an AE that precluded RC. However, interesting, the 4 patients that refused cystectomy are doing well.
Looking at outcomes, the median time to surgery was 18.5wks from registration and 5.3wks from the last dose. Adding pembrolizumab did not delay cystectomy compared to chemotherapy alone.
This next table is the take-home outcomes:
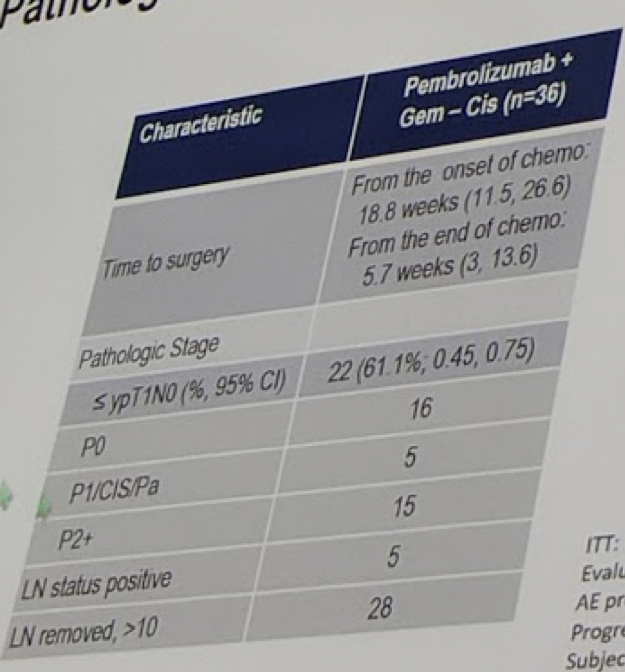
While the primary outcome was downstaging to <T2 disease (22 patients, 61%), the CR rate (pT0) was 16 patients (40%). Pre-treatment PD-L1 status was not associated with a response.
Secondary endpoints were CSS and OS, which at 18 months, were 90% and 81%, respectively.
Invited Discussant
Dr. Rob Jones, in his discussion, made the following points:
- The CR rate (pT0 status) was not much different from chemotherapy alone – 38% in the MVAC studies.
- Adding pembrolizumab appears to have manageable toxicity
- Adding pembrolizumab does not appear to delay surgery more than chemo alone
- The pembrolizumab doses were limited to 5 doses – no adjuvant therapy was given. This may not have affected RC pathology outcomes but may affect survival results down the line.
- This is a small population size and lacks randomization – with its inherent risks
- There remains uncertainty about the primary endpoint of downstaging to <pT2 rather than CR.
A confirmatory phase III RCT is planned. There are also other ongoing trials of ICI monotherapy, combination therapies, and sequential therapies to help address this question!
Presented by: Christopher J. Hoimes, DO, Hematology and Oncology, Assistant Professor, Medicine, CWRU School of Medicine
Cleveland, Ohio, US
Invited Discussant: Rob Jones, Professor, The University of Glasgow, Glasgow, Great Britain
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, twitter: @tchandra_uromd, @TjuUrology at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


