He began by highlighting the rationale for the use of PARP inhibitors (such as olaparib) in this disease space based on BRCA-related synthetic lethality with poly ADP ribose polymerase (PARP) inhibition.

This is particularly relevant in advanced prostate cancer given data from Drs. Pritchard, Robinson, and colleagues demonstrating the prevalence of both germline and somatic DNA repair mutations. However, Dr. Fizazi emphasized that DNA repair defects in the context of mCRPC are not restricted to young men or those with a family history with a relative preponderance of somatic mutations compared to germline.
Notably, the efficacy of existing systemic therapies appears independent of DNA damage repair status, with Dr. Fizazi highlighting recent data presented at ESMO 2020 which showed comparable responses to cabazitaxel among those with DDR mutations and without.
The rationale for olaparib in this disease space came from work from Dr. Mateo who demonstrated that the response to olaparib was dependent upon the presence of DDR mutations.
Subsequently, the PROfound study recruited men with metastatic castrate-resistant prostate cancer who had progressed on previous abiraterone acetate or enzalutamide administered at the time of non-metastatic castrate-resistant prostate cancer or at the time of metastatic castrate-sensitive prostate cancer, many of who had previously been treated with taxane chemotherapy. The investigators then used an investigational assay based on the FoundationOne CDx to identify alterations in one of 15 pre-specified genes involved in homologous recombination repair (BRCA 1/2, ATM, BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L).
The authors used biomarker-driven stratification to derive two study cohorts: Cohort A had alterations in BRCA1, BRCA2, or ATM while Cohort B had alterations in any of the other 15 included genes. In both cohorts, patients were randomized 2:1 to Olaparib vs. abiraterone or enzalutamide. Within each biomarker strata, randomization was stratified based on prior taxane use and measurable disease burden (according to RESIST 1.1 criteria).
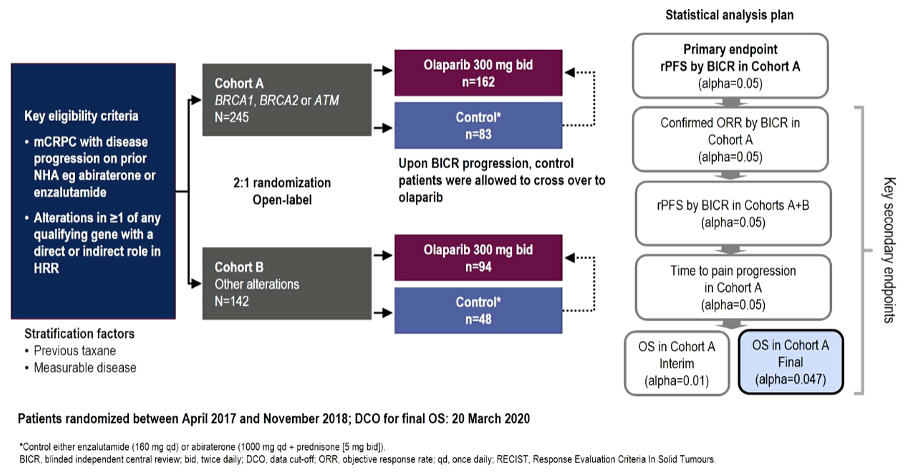
With respect to the primary outcome of radiographic progression-free survival in Cohort A, the study was strongly positive with a hazard ratio of 0.34 (95% confidence interval 0.25 to 0.47). There was a similar benefit in time to pain progression and even more profound benefit in objective response rate. In updated analyses presented at ESMO 2020 and subsequently published, there was an improvement in overall survival based on the intent to treat population (hazard ratio 0.69, 95% confidence interval 0.50 to 0.97) and in cross-over adjusted analyses (hazard ratio 0.42, 95% confidence interval 0.19 to 0.91).
However, Dr. Fizazi highlighted that no overall survival benefit of olaparib was apparent in Cohort B, among patients with mutations in 12 genes other than BRCA1, BRCA2, and ATM. He highlighted that gene-by-gene analyses suggested that the overall survival benefit was restricted to men with BRCA alterations.
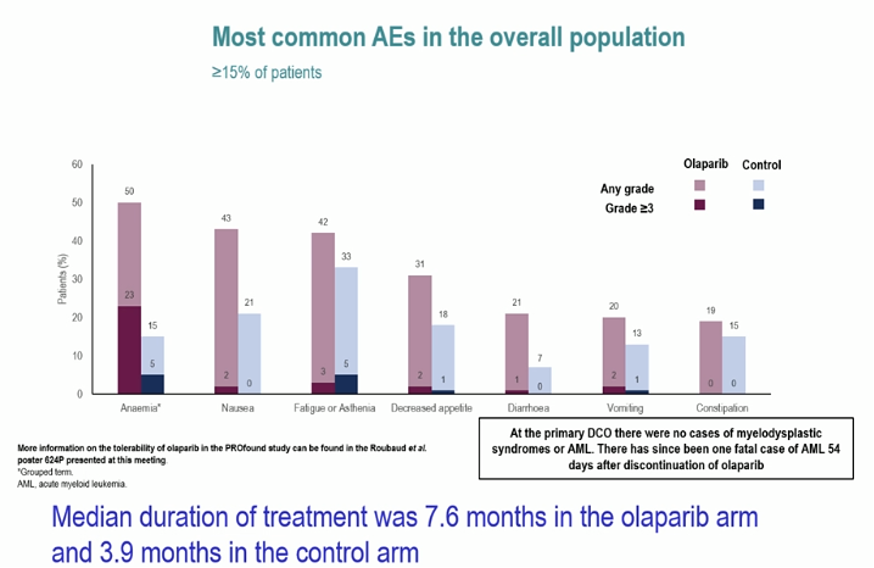
Dr. Fizazi then highlighted the toxicity profile of this approach, including rates of anemia, nausea, diarrhea, and fatigue which were higher in the olaparib arm. However, he emphasized that these differences should be interpreted in the context of nearly twice as long therapy in the olaparib arm.
He concluded by highlighting that this study represents the first positive biomarker-selected trial in prostate cancer. These data have led to the approval of olaparib in this disease space but also have highlighted an urgent need for more widespread DDR testing.
Presented by: Karim Fizazi, MD, Ph.D. Professor of Medicine of Institut Gustave Roussy, (IGR) in Villejuif, France.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
NEJM: Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer.
Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Can Be Preselected to Maximize Benefit of Olaparib - Maha Hussain
ESMO Virtual Congress 2020: Final Overall Survival Analysis of PROfound: Olaparib vs Physician’s Choice of Enzalutamide or Abiraterone in Patients with mCRPC and Homologous Recombination Repair Gene Alteration
The Role of FoundationOne® Liquid CDx in mCRPC - Oliver Sartor


