(UroToday.com) In the fourteenth session of the 2022 International Kidney Cancer Symposium (IKCS): Europe meeting focusing on treatment approaches for patients with non-clear cell renal cell carcinoma (nccRCC), Dr. Gabriel Malouf discussed treatment approaches for patients with MiT family translocation renal cell carcinomas (RCC).
He began by first discussing the biology of this relatively rare subset of RCC, which was first introduced into the WHO classification of kidney cancer as a distinct entity in 2004. While comprising only 1% of all RCCs, it accounts for half of kidney carcinomas in children and approximately 15% in young adults less than 45 years of age. Further, there is a female predominance.
Molecularly speaking, these tumors have translocations in members of the MiT family of transcription factors including TFE3 and TFEB. Numerous different translocation partners have been described for each of these, though the underlying molecular mechanisms for carcinogenesis remain unclear. However, broadly speaking, these transcription factors are involved in processes important for cell growth regulation and cell metabolism regulation.

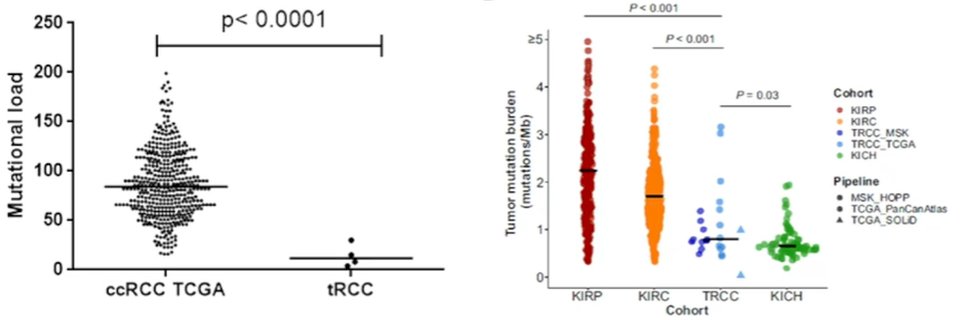
Initial work examining treatment approaches for these patients was published approximately a decade ago, at which time the diagnosis was made by IHC, without FISH being available. In two small cohorts from France (n=21) and the United States (n=15), responses to VEGF-tyrosine kinase inhibitors was relatively poor with median overall survival of 14 months. More recently, in the immunotherapy era, response to first-line TKI and second-line immune checkpoint inhibitor were both relatively poor with progression free survival of 3 months or less, suggesting progression at the first scan. Part of the reason that these tumors may respond poorly to immunotherapy is that they have a relatively low tumor mutational burden compared to other RCC subtypes, though TMB has not been shown to be predictive of immunotherapy response in RCC more broadly.
In terms of gene expression signature profiling, while the numbers are small (n=15), all patients with MiT family translocation renal cell carcinomas profiled were in cluster 5 (proliferative) with low angiogenesis and low PD-L1 expression. Interestingly, based on this, there is some evidence of response to the combination of atezolizumab and bevacizumab, though responses to sunitinib were poor.
Considering the potential for immunotherapy, Dr. Malouf highlighted that these tumors are immunologically cold without evidence of significant immune infiltration, apart from a subset of MED15-TFE3 fusion tumors.
Interestingly, in contrast to these relatively sobering results, he showed data from a recent retrospective cohort of 52 patients demonstrating relatively promising results from the use of cabozantinib in this cohort with median progression free survival of 6.8 months and median overall survival of 18 months. Further, this treatment approach had a complete response in 2 patients with a clinical benefit in 46%.
While these tumors appear to have primary resistance to a combination dual immune checkpoint inhibitor regime, Dr. Malouf suggested that a combination approach utilizing immune checkpoint blockade along with a tyrosine kinase inhibitor (particularly cabozantinib) may be more efficacious, though the number of patients treated with this approach in the published literature remains small.

In conclusion, Dr. Malouf highlighted that translation renal cell carcinoma with a heterogeneous disease with many different translation partners resulting in different clinical outcomes and tumor microenvironments. However, they are predominantly immunologically cold. To advance treatment options, he emphasized that prospective, molecularly-driven trials are needed.
Presented by: Gabriel Malouf, MD, PhD, University of Strasbourg

