- prognosis in the second-line space in the era of first-line immune checkpoint based combination treatments;
- the question of treatment intensification beyond the first-line setting;
- whether all agents can be considered equivalent in this space;
- what novel treatment approaches may be coming along.
Dr. Albiges then considered the question of whether there is a rationale for treatment intensification beyond first-line therapy. Under this umbrella, she first highlighted the question of whether we can cure patients beyond first-line combination treatment failure. There have been a number of trials assessing the use of ipilimumab as an anti-CTLA4 based rescue strategy in IO/TKI pre-treated patients. Unfortunately, objective response rates for this approach were poor.

She then assessed the question of the role of sustained PD-1 inhibition at the time of initiating second line therapy. This is being assessed in two ongoing trials (CONTACT-03 and TiNivo-2) which will compare the role of TKI alone compared to TKI with immune checkpoint inhibitor.
In contrast to sustained PD-1 inhibition, she then addressed the question of the role of PD-1 rechallenge. The data in this setting comes from “small but important” retrospective studies. These show that the overall response rate was 23%. She suggested that patients who had progressive disease are unlikely to benefit from rechallenge whereas those with initial response who subsequently go on a drug holiday and then have progression may have some benefit.
Citing a 2021 publication from Dr. Lee, she highlighted “intriguing” data assessing the role of Lenvatinib and pembrolizumab among a patient population that is primarily pre-treated with immunotherapy based treatment regimes. Nearly 56% of patients had objective response with this combination treatment approach. Thus, this, she suggested, is a promising approach that warrants randomized evaluation.
Dr. Albiges then discussed a potential role for early treatment intensification among patients who have stable disease at 3 months following initial dual immune checkpoint inhibition with nivolumab and ipilimumab, as is being assessed in the PDIGREE study. This trial adopts an adaptive design: in patients who have complete response, maintenance nivolumab (as is standard of care) is recommended; in patients with progressive disease, a switch to cabozantinib is mandated; however, of greatest interest, in those with stable disease, patients will be randomized to receive nivolumab maintenance (standard) or nivolumab with cabozantinib (intensified therapy).
Dr. Albiges then considered the question of whether all tyrosine kinase inhibitors are equivalent after both dual IO-IO and IO-TKI therapy. She highlighted data from the IMDC showing a variety of treatment patterns in real-world practice.
However, to inform the question of whether different agents have different efficacy, we are limited to small retrospective studies, which (acknowledging their limitations) don’t seem to show a difference between sunitinib, axitinib, pazopanib, and cabozantinib.
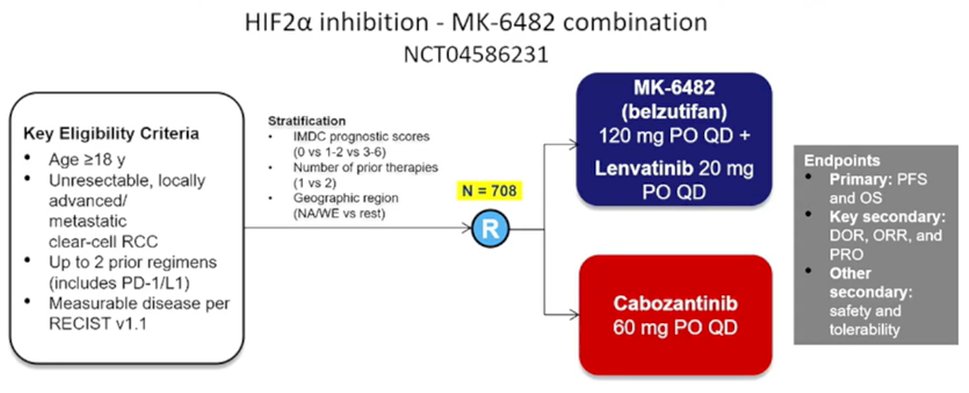
Finally, she discussed potential new treatment approaches which are coming, first highlighting the role of belzutifan as a HIF2alpha inhibitor. In phase I/II data, belzutifan monotherapy has shown promising efficacy with an objective response rate of 25% among patients with a median of 3 prior lines of therapy. A randomized comparison of belzutifan and everolimus in this setting is currently fully accrued and awaiting read out. However, Dr. Albiges suggested that belzutifan might be better suited to a combination therapy approach which is also currently being addressed in a randomized trial comparing belzutifan and lenvatinib compared to cabozantinib, among patients with up to two prior lines of therapy.
In closing, she noted the importance of multimodal treatment approaches including radiation therapy and focal treatments; bone targeting agents; and the potential of therapies to modify the host microbiome. Further, she emphasized that there is no cure yet beyond first-line immunotherapy based treatment regimes and that there is no evidence favouring a particular TKI. What randomized data should be favoured, sequence trials are long and difficult to conduct so she highlighted the value of real-world data.
Presented by: Laurence Albiges, MD, PhD, Medical Oncology, Institut Gustave Roussy, Villejuif, France


