(UroToday.com) In the sixth session of the 2022 International Kidney Cancer Symposium (IKCS): Europe meeting focusing on later lines of systemic therapy in advanced kidney cancer, Drs. Camillo Porta and Laurence Albiges debated the approach to second-line therapy in metastatic renal cell carcinoma (mRCC). Dr. Porta presented first, emphasizing evidence-based second line treatment approaches.
He first used two patient cases to highlight the treatment scenarios that are most relevant: in the post-immunotherapy setting and in the post-TKI setting. Beginning with the second of these, Dr. Porta considered available data for second-line treatment choice in the post-TKI setting. While immunotherapy based treatment regimes have become relatively widespread, he emphasized that this is still a credible scenario as first-line use of TKI monotherapy remains fully justified both in IMDC good risk patients and in countries in which immune-based chemotherapy options are not available or reimbursed.
In this setting, he suggested that treatment choice is relatively easy, given the availability of randomized controlled trials. Highlighting data from CheckMate-025 and METEOR, respectively, he emphasized that nivolumab and cabozantinib are his two preferred treatment approaches.
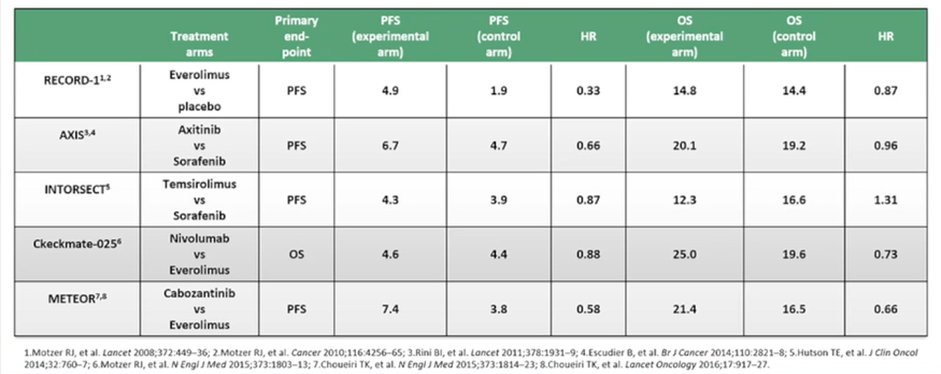
He further highlighted, based on data from Drs. Rini and Atkins, that angiogenesis remains a key driver and key pathophysiologic event for patients, even following progression after an initial course of TKI. This work has shown that reactivation of angiogenesis is present at progression following initial TKI treatment. Thus, the use of cabozantinib remains both evidence supported and rationale. Choosing between cabozantinib and nivolumab, he suggested that there is minimal difference in overall survival between these treatment approaches, based on an indirect comparison. However, cabozantinib acts relatively early while nivolumab is “more active later on during treatment” which may influence treatment choice.

Dr. Porta then moved to discuss second-line treatment approaches for patients who have previously received immune-based combination treatments. Dr. Porta began that any VEGFR-TKI that was not used in the initial first-line treatment combination may be considered and may be efficacious. Supporting this approach, he highlighted data, emphasizing that much of this is derived from retrospective studies of relatively small patient cohorts. Further, some of these are following immunotherapy monotherapy. Broadly speaking, he suggested that TKIs were similarly effective to mTOR inhibitors (while there is limited data for the role of mTOR inhibitors) with an average objective response rate of 31% and progression-free survival of 7.4 months. While data are limited to date, he suggested that belzutifan may be particularly efficacious in this setting though it is not yet indicated.
Dr. Porta then considered the “counterintuitive” question of sequencing immune checkpoint inhibitors. In this context, there are three different randomized controlled trials (TITAN-RCC, OMNIVORE, and HCRN GU16-260) that suggest relatively limited response rates (average objective response rate of 24%) and few complete responses. As above, the average median overall survival across trials was approximately 7.4 months. However, based on data from Lee and colleagues, he suggested that a combination IO/TKI approach (in this case, Lenvatinib and pembrolizumab) may be particularly efficacious.
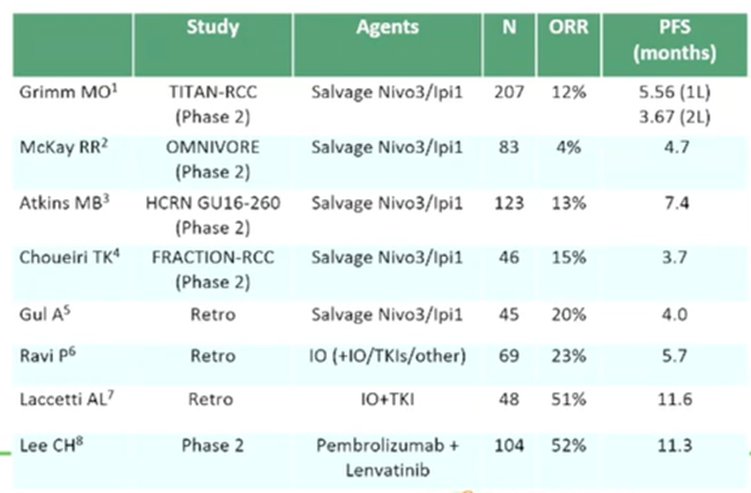
Taken together, he suggested that immunotherapy in second line, following immunotherapy in first line, has limited activity though there are some striking responses. Interestingly, second-line activity appears to be somewhat independent of prior response to immunotherapy.
Concluding, Dr. Porta highlighted that there is Level 1 evidence supporting cabozantinib or nivolumab in the second line setting following first-line TKI monotherapy. Following first-line immunotherapy-based combination treatment, he suggested that any TKI not used in first-line “makes sense” though activity and efficacy are “expected not to be striking” and the level of evidence for this recommendation is only 3. Further, limitations imposed by regulatory and reimbursement agencies must also be considered. He further emphasized that immunotherapy following immunotherapy remains relatively poorly explored with only level 4 evidence to guide. Thus, while other immune checkpoint inhibitors apart from anti-PD-1/PD-L1 and anti-CTLA4 should be explored, these are not yet clinically relevant. Similarly, belzutifan appears to be a promising approach though much more data is required.
Presented by: Camillo Porta, MD, University of Bari Aldo Moro, Italy

