(UroToday.com) The 2023 European Association of Urology (EAU) annual congress held in Milan, Italy between March 10th and 13th, 2023 was host to a plenary session addressing the “right management” of prostate cancer patients in the early detection and active surveillance (AS) settings.
Dr. Matthew Cooperberg discussed whether a genetic test of biopsy specimens was needed to promote active surveillance in Grade Group 2 (GG2) prostate cancer patients.
Dr. Cooperberg began by emphasizing that GG2 disease is the correct cohort of patients to consider the application of genetic testing to, when considering active surveillance, as patients with low-risk, GG1 should be uniformly managed with active surveillance. Active surveillance rates for GG1 disease have significantly improved in the US, rising from about 30% in 2014 to almost 60% in 2021. However, there remains significant heterogeneity in the appropriate use of active surveillance in GG1 patients, both at the practice- and physician-levels, even within the same practice.

How do the numbers compare in the GG2 cohort? A study by Loeb et al. from a Swedish population-based registry demonstrated that AS rates for GG2 patients are approximately 25%. Conversely, in the US, these figures depend on the setting this question is evaluated in. Within the MUSIC cohort from Michigan, these figures have increased since 2014, with the introduction of MUSIC initiatives, from about 5% to 25%.
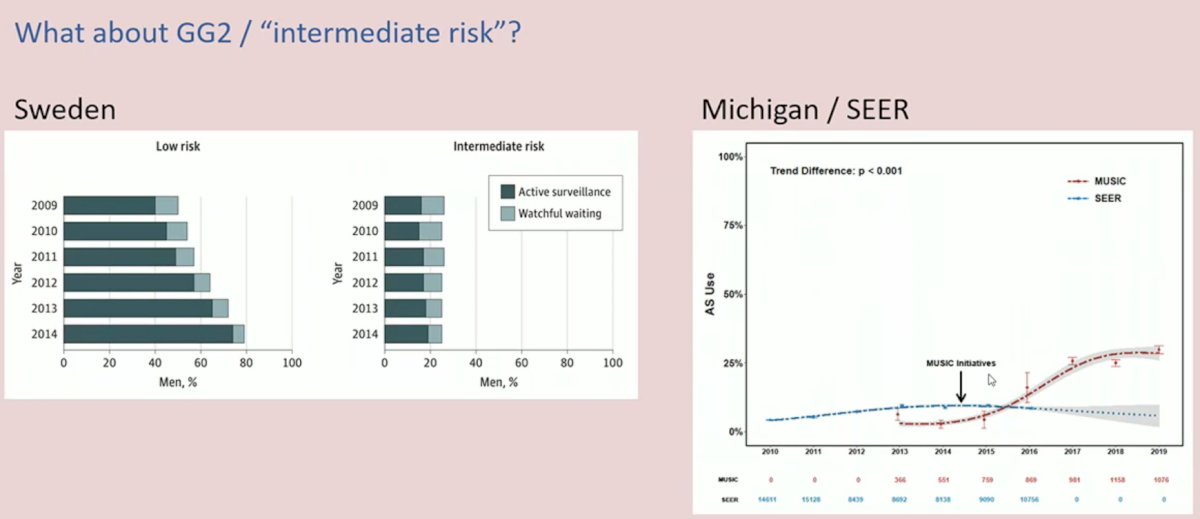
One important point to highlight is that GG2 is an extremely heterogenous disease state. As such, tools to further stratify this cohort of patients is critical for the appropriate selection of active surveillance candidates.

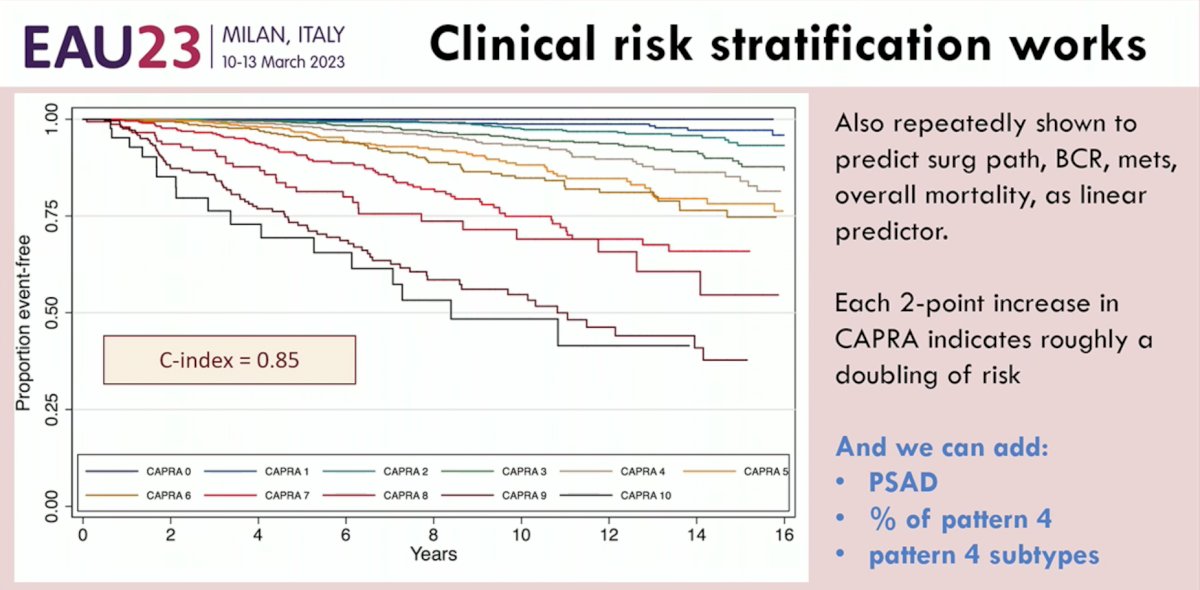
Before we consider genetic testing, we must emphasize that clinical risk stratification tools do work in this setting. The CAPRA score published in 2009 by Cooperberg et al. demonstrated excellent granularity with a concordance index of 0.85 for event-free survival.2 This nomogram has been shown to reliably predict surgical pathology, biochemical recurrence, metastases, and overall mortality outcomes. Each two-point increase in the CAPRA score indicates roughly a doubling of these risks. A more nuanced approach to the CAPRA score could involve combining this tool with other clinical, widely available parameters, including PSA density, % of pattern 4, and pattern 4 subtypes.
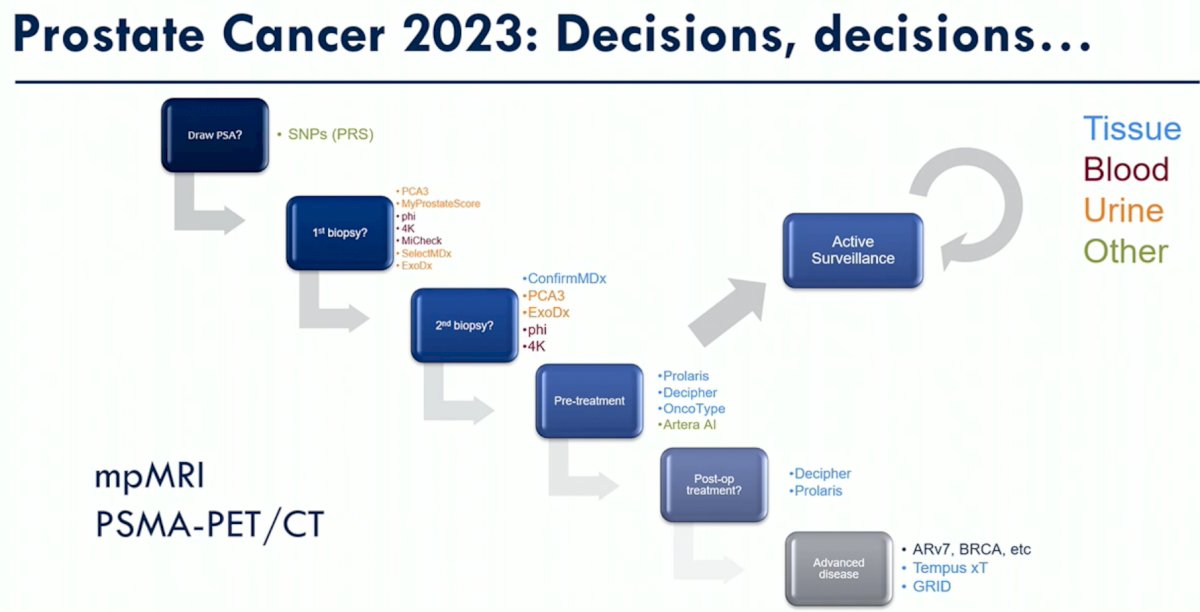
Numerous genetic-based tools have become commercially available in multiple settings, including the 1st biopsy, repeat biopsy, pre-treatment decision-making, post-operative, and advanced disease settings. Novel AI-based models by Artera® AI have also emerged at the forefront of such decision aid tools.
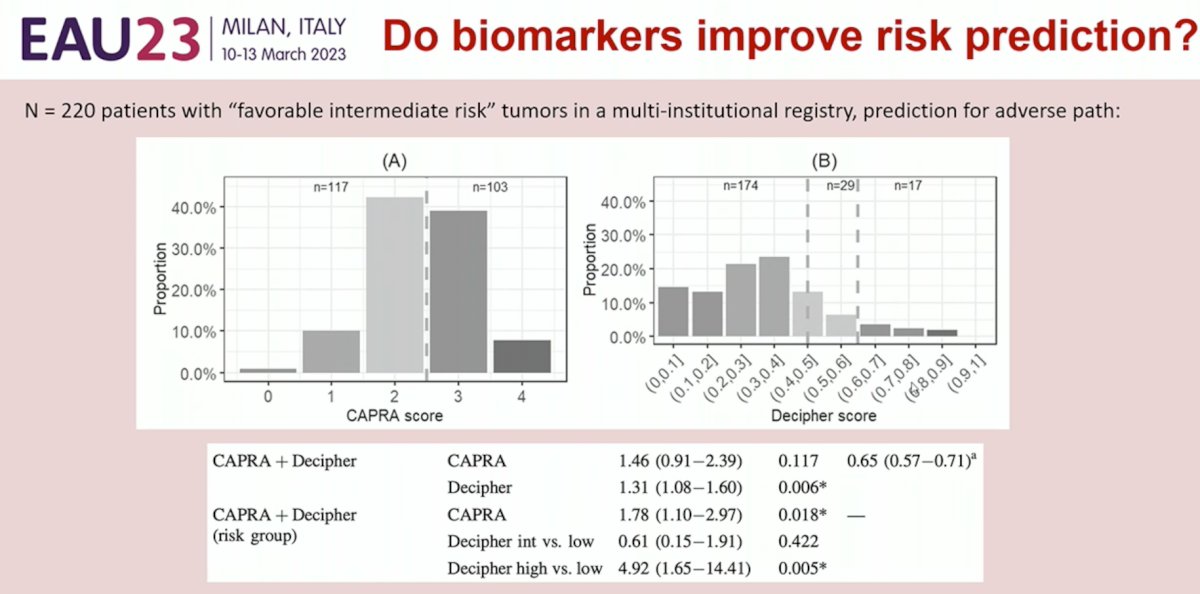
Do biomarkers improve risk prediction? The answer appears to be yes. But do these genetic tests offer additional, independent information above and beyond what clinical stratification tools provide? Again, the answer appears to be yes. Multivariable analyses accounting for the CAPRA score have demonstrated that results from Decipher® provide additional prognostic information, independent of CAPRA score. Furthermore, these tests provide additional granularity that is not possible with current clinical stratification tools.
The current ASCO guidelines state that: “ Commercially available molecular biomarkers (i.e. Oncotype Dx Prostate, Prolaris, Decipher, and ProMark) may be offered in situations in which the assay results, when considered as a whole with routine clinical factors, is likely to affect management. Routine ordering for molecular biomarkers is not recommended (Type: Evidence based; Evidence quality: Intermediate; Strength of recommendation: Moderate).”
But do biomarkers actually improve health outcomes secondary to selection of active surveillance versus treatment? The answer remains unclear at the current time.
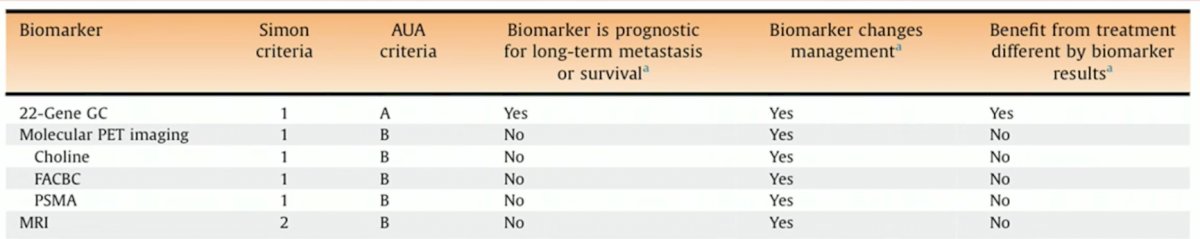
While PET-based imaging is emerging as a potential tool or selection of GG2 active surveillance candidates, to date, information from these images is not prognostic of long-term metastases or survival. Conversely, the 22-gene Decipher® genomic classifier appears to be prognostic of such outcomes, as demonstrated in the table below.
Dr. Cooperberg concluded his presentation by asking: how should these results be communicated? The clinician and the patient are both faced with an “avalanche” of clinical and genomic classifiers, and how such tools should be used in practice, as of 2023, remain unclear.
Presented by: Matthew R. Cooperberg, MD, MPH, Professor of Urology and Epidemiology & Biostatistics and Helen Diller Family Chair in Urology at the University of California, San Francisco, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Association of Urology (EAU) Annual Meeting, Milan, IT, Fri, Mar 10 – Mon, Mar 13, 2023.
References:- Cooperberg, et al. Time Trends and Variation in the Use of Active Surveillance for Management of Low-risk Prostate Cancer in the US. JAMA Netw Open, 2023. 6(3):e231439.
- Cooperberg, et al. Risk Assessment for Prostate Cancer Metastasis and Mortality at the Time of Diagnosis. JNCI, 2009. 878–887


