(UroToday.com) The 2022 EAU annual meeting featured a game-changing session, including a presentation by Dr. Noel Clarke discussing exploratory endpoints from PROpel, a phase III trial of abiraterone + olaparib versus abiraterone + placebo in first-line mCRPC. There is a need to improve patient outcomes in the first-line mCRPC treatment setting. Previously, the PROfound trial of olaparib monotherapy demonstrated significantly longer radiographic progression free survival (rPFS) and overall survival (OS) in patients with mCRPC post-NHA treatment with selected HRR gene alterations (BRCA1, BRCA2, and ATM).1,2 Furthermore, a phase II trial combining abiraterone + olaparib versus abiraterone + placebo has shown prolonged rPFS in patients with mCRPC following docetaxel and irrespective of HRR mutational status (HR 0.65, 95% CI 0.44-0.97).3 The PROpel primary results have been published,4 demonstrating a significant improvement in rPFS with abiraterone + olaparib over abiraterone + placebo as first-line treatment in mCRPC, and the presentation by Dr. Clarke at EAU 2022 adds additional efficacy data from exploratory endpoints of the trial.
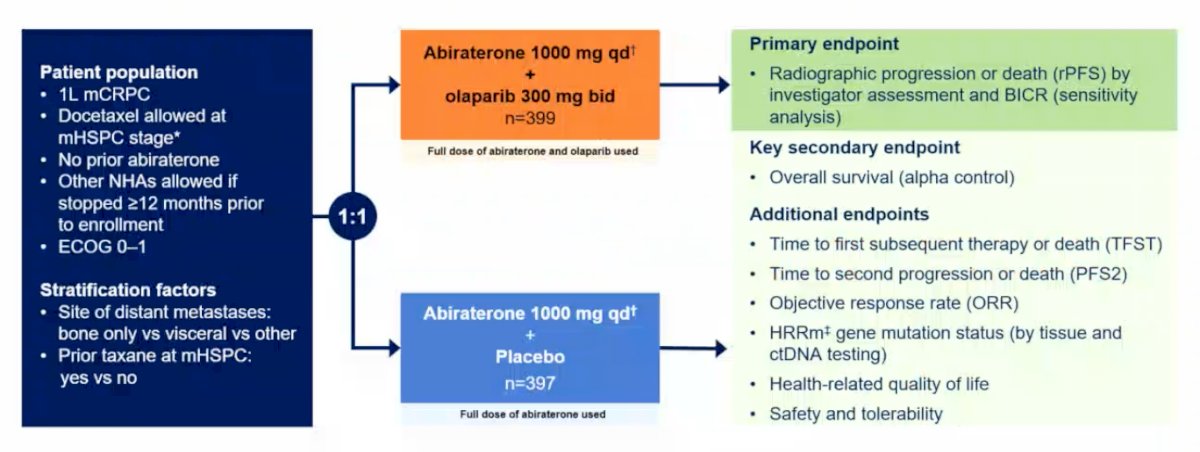
PROpel is a global, randomized, double-blind phase III trial randomizing men with mCRPC to first-line abiraterone + olaparib vs abiraterone + placebo in a 1:1 fashion. The primary endpoint is rPFS by investigator assessment and by blinded independent review, whereas the key secondary endpoint is overall survival. Additional key endpoints include: (i) time to first subsequent therapy or death (TFST), (ii) time to second progression or death (PFS2), (iii) objective response rate (ORR), (iv) HRR gene mutation status (by tissue and ctDNA testing), (v) health-related quality of life, and (vi) safety and tolerability:
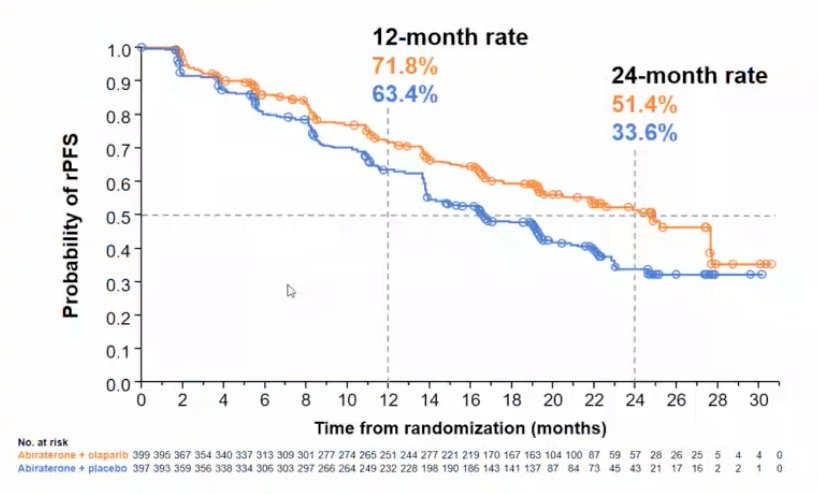
The median rPFS for abiraterone + olaparib was 24.8 months vs 16.6 months for abiraterone + placebo (HR 0.66, 95% CI 0.54-0.81), demonstrating a 34% reduction in the risk of radiological progression or death with abiraterone + olaparib:
These results were consistent by blinded independent central review: abiraterone + olaparib median rPFS 27.6 months vs 16.4 months for abiraterone + placebo (HR 0.61, 95% CI 0.49-0.74). Additionally, the rPFS benefit was observed across all pre-specified subgroups, and the global interaction was not significant:
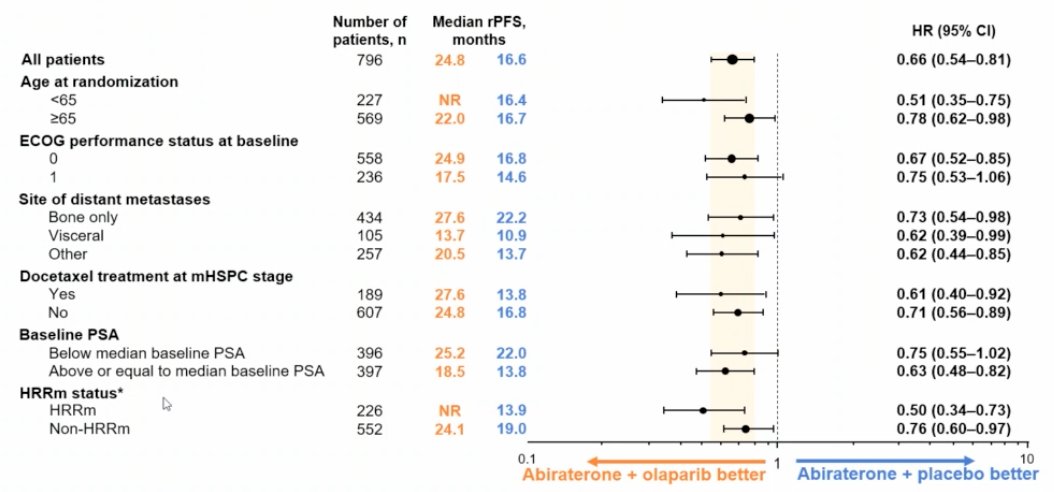
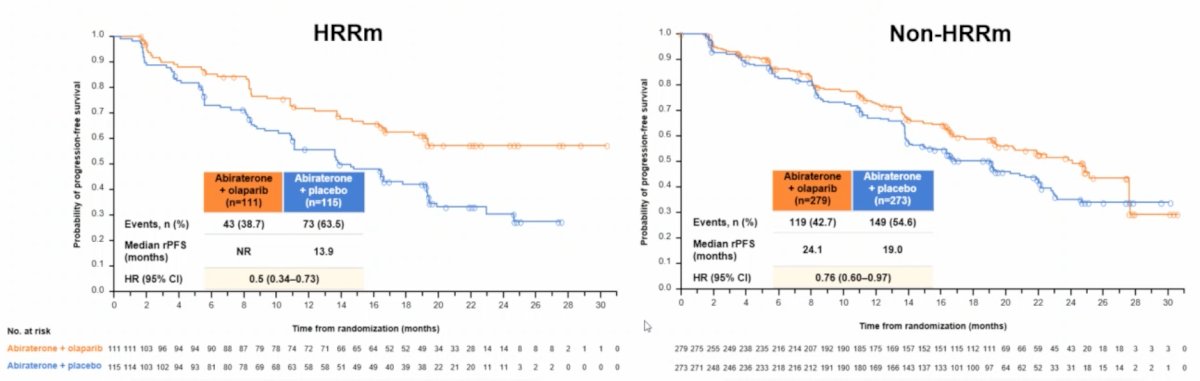
Among HRR mutation patients, the median rPFS for abiraterone + olaparib was not reached vs 13.9 months for abiraterone + placebo (HR 0.50, 95% CI 0.34-0.73). Furthermore, among non-HRR mutation patients, the median rPFS for abiraterone + olaparib was 24.1 months vs 19.0 months for abiraterone + placebo (HR 0.76, 95% CI 0.60-0.97):
Overall survival (28.6% maturity) showed a trend towards improvement with abiraterone + olaparib (HR 0.86, 95% CI 0.66-1.12), in addition to TFST (HR 0.74, 95% CI 0.61-0.90) and PFS2 (HR 0.69, 95% CI 0.51-0.94) results supporting longer-term benefit with abiraterone + olaparib. With regards to PSA dynamics, there was a 10% higher confirmed PSA response rate with abiraterone + olaparib, and the median time to PSA response (abiraterone + olaparib not reached vs 12.0 months olaparib + placebo) favored the abiraterone + olaparib arm (HR 0.55, 95% CI 0.45-0.68). The adverse event profile was consistent with the known toxicity profiles for the individual drugs:
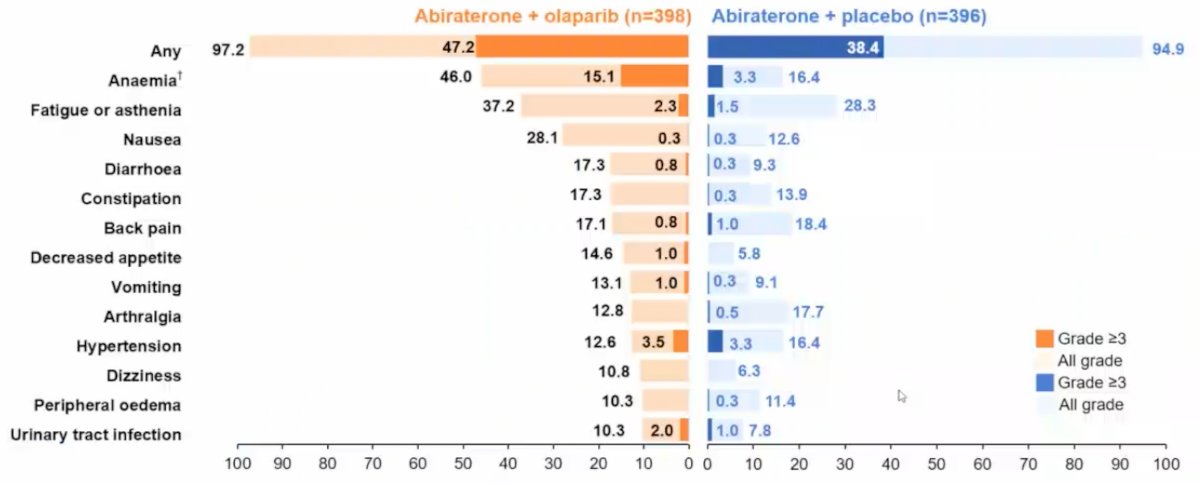
Dr. Clarke concluded his presentation discussing exploratory endpoints from PROpel with the following take-home messages:
- Abiraterone + olaparib produced a statistically significant improvement in rPFS (HR 0.66, 95% CI 0.54-0.81) compared with abiraterone + placebo in first-line mCRPC
- Secondary endpoints support the superiority of the combination of abiraterone + olaparib compared with abiraterone + placebo
- The safety profile of abiraterone + olaparib was consistent with the profiles for the individual drugs, enabling the majority of patients to remain on treatment
- PROpel is the first phase III study to demonstrate the clinical benefits of abiraterone + olaparib for patients in the first-line mCRPC setting enrolled irrespective of HRR mutational status
Presented by: Noel W. Clarke, MBBS, FRCS, ChM, The Christie Hospital, Manchester, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomized, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018 Jul;19(7):975-986..
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence 2022.EVIDoa2200043.


