While neoadjuvant chemotherapy is the standard of care for bladder cancer, neoadjuvant chemotherapy trials in the management of UTUC with radical nephroureterectomy (RNU) have not yielded comparable results. More recent data from the POUT study (Birtle et al. ASCO GU 2018) is the latest and strongest evidence of the benefit of perioperative (albeit adjuvant) systemic therapy for these high-risk patients.
In this multi-center, phase II randomized trial entitled URANUS, Neo-adjUvant veRsus AdjuvaNt chemotherapy in upper tract Urothelial carcinoma: A feaSibility phase II randomized clinical trial, the authors aim to compare neoadjuvant vs adjuvant chemotherapy in patients eligible for radical nephron-ureterectomy (RNU) or distal ureterectomy (DU) and lymph node dissection (LND) in patients with cT2-cT4 and cN0-N1 M0 (high-risk) UTUC. Clinicaltrials.gov number: NCT02969083.
Study Design and Protocol:
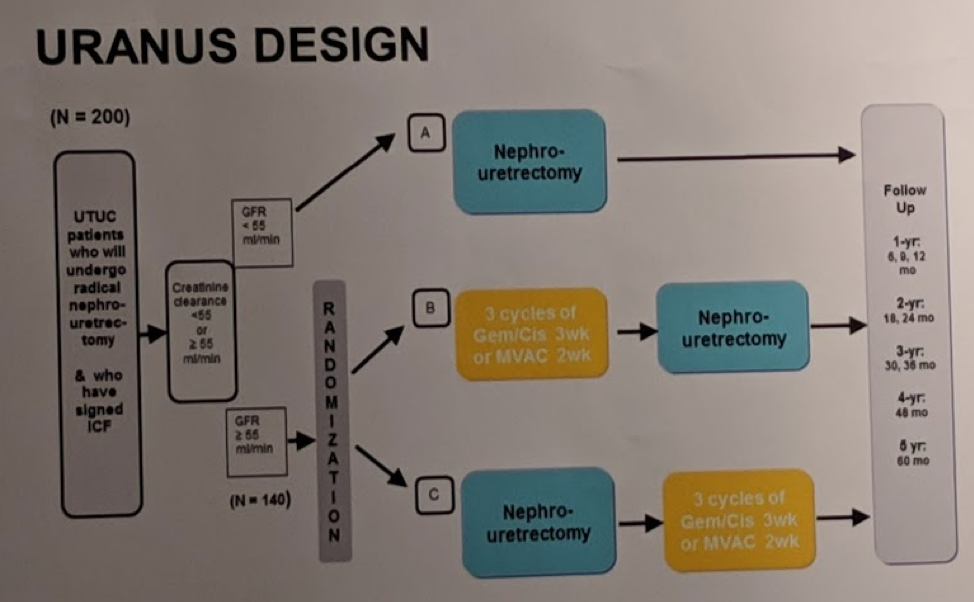
Randomized prospective phase II study to collect real-world data in Upper Tract Urinary Transitional Cell Carcinoma (UTUC). There will be 3 arms to the study, based on eligibility for chemotherapy.
Patients who fulfill inclusion criteria and have a GFR ≥ 55 ml/min will be randomized to either neoadjuvant (Arm B) or adjuvant (Arm C) chemotherapy consisting of 3 cycles of gemcitabine/cisplatin (Gem/Cis). Centers with experience with MVAC in UTUC, may consider 3 cycles of dose-dense MVAC.
Patients who don’t fulfill inclusion criteria for treatment randomization or have GFR <55 ml/min will undergo RNU only (Arm A)
Full protocol can be seen below:
Inclusion/Exclusion Criteria:
Adult patients aged ≥ 18 with PS 0-1, cytological or histological proven upper tract UCC staged cT2-cT4 and cN0-N1 MO are eligible for the study.
Patients with the following features will be excluded: concomitant non-muscle invasive bladder cancer.
Outcomes:
Primary end points:
- Assess the proportion of UTUC patients with adequate renal function and fit to receive either neo- or adjuvant cisplatin-based chemotherapy and planned dose treatment and also able to start and finalize three courses of planned chemotherapy
- Assess 1- and 2-year Disease Free Survival (DFS)
- Assess 1- and 2-year Overall Survival (OS)
- Assess 1- and 2-year Cancer-Specific Survival (CSS)
Sample Size Calculation and Current Status:
The study is expected to enroll ~200 patients. Of these, ~140 are through to be randomized to either arm B or C (70 in each arm).
Presented by: Juan Palou, MD, PhD, FEBU, Chairman, Department of Urology, Fundació Puigvert, Barcelona, Spain
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @TjuUrology) at the 34th European Association of Urology (EAU 2019) #EAU19 conference in Barcelona, Spain, March 15-19, 2019.


