(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a meeting of the EAU Section of Oncologic Urology (ESOU) and a presentation by Dr. Riccardo Campi discussing the future of immunotherapy in localized kidney cancer. Dr. Campi started by noting that the EAU guidelines state that patients should be offered surgery to achieve a cure in localized renal cell cancer (strength rating: strong), thus for non-metastatic RCC is there a need for multimodal treatment? Dr. Campi states that it is important to predict the puzzle of the future, with several key points:
- Understanding the clinical unmet need
- Knowing the past (of adjuvant/neoadjuvant treatment for RCC)
- Riding the wave of the present
- Having the courage to imagine, explore (and guide) the future
- Being critical and wise (know your limits)
- Making it real, together, for patients
Dr. Campi emphasized that the past utilization of adjuvant antiangiogenic agents showed no overall survival benefit, as highlighted by the following trials:
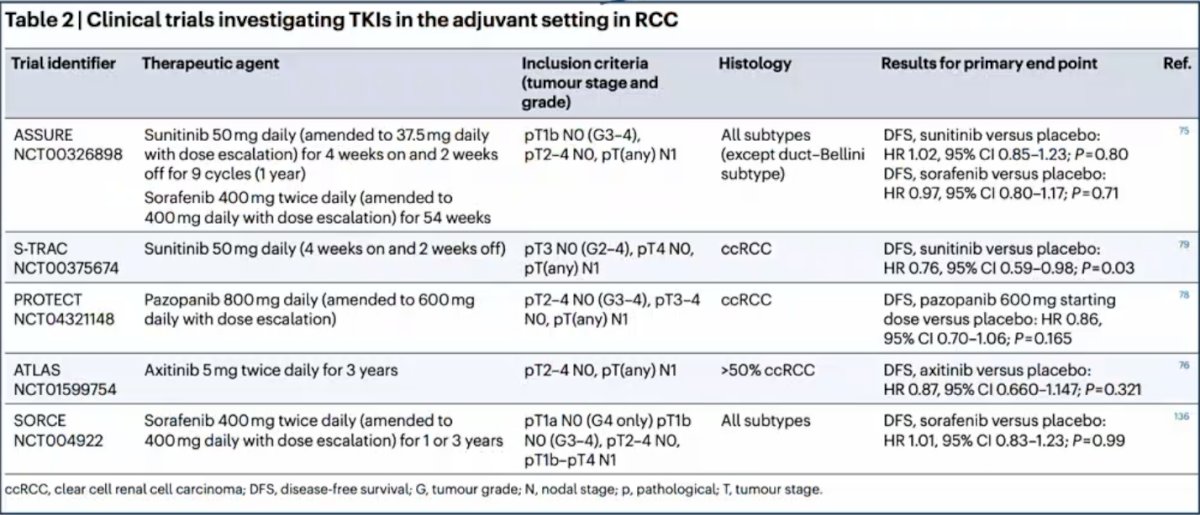
Over the last decade, the evolution of systemic therapy for mRCC has been dramatic, particularly as we look at the data from 2018 that has now translated into the EAU guidelines in 2023:

Dr. Campi notes that there are different approaches to integrating immunotherapy in the multimodal treatment of high risk RCC as depicted below:
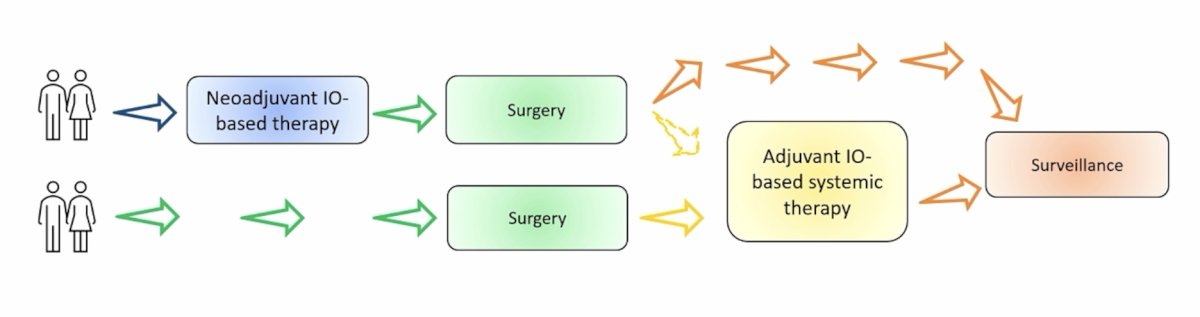
Dr. Campi states that adjuvant immunotherapy for RCC has conflicting evidence when we look at the positive outcome of KEYNOTE-5641 and the negative trials of PROSPER, CheckMate 914, IMmotion010. Potential reasons for these discrepancies include:
- Heterogeneous populations
- Trial design
- Differences in treatment-related toxicity
- Random chance
- Effect of PD1 versus PDL1 inhibitors on the metastatic cascade
- Length of follow-up
- Length of treatment
- Method for assessment of disease free survival
- Histology, stage, the percent of M1NED, the percent of sarcomatoid RCC
As such, the EAU guidelines suggest the following when considering adjuvant immunotherapy for RCC:
- Discuss the contradictory results of the available adjuvant ICI trials with patients to facilitate shared decision making (Strength rating: strong)
- Inform patients about the potential risk of overtreatment and immune-related side effects if adjuvant therapy is considered (Strength rating: strong)
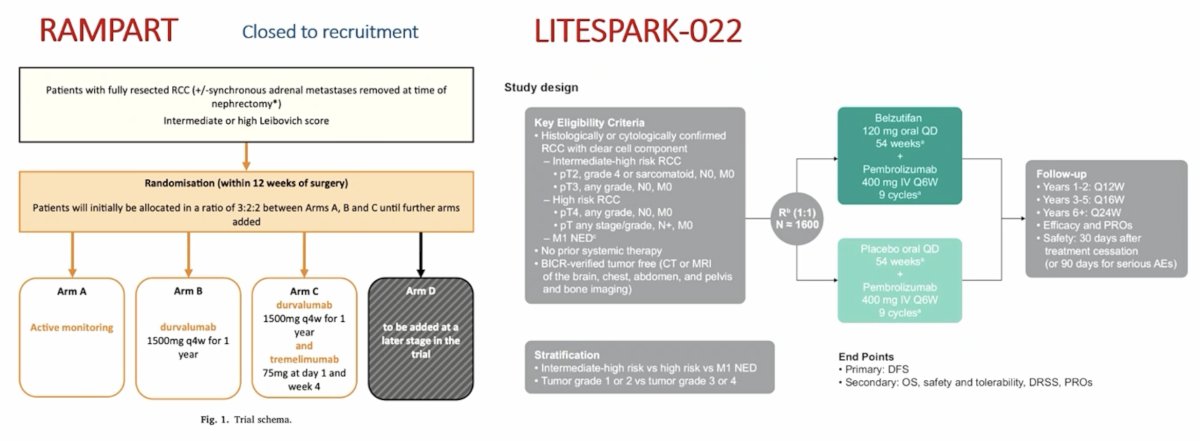
Of note, there are several other adjuvant immunotherapy trials yet to report data, including the RAMPART trial (closed to recruitment) assessing active monitoring vs durvalumab vs durvalumab + tremelimumab, and the LITESPARK-022 trial assessing belzutifan + pembrolizumab versus placebo + pembrolizumab:
Dr. Campi notes that there is also a rationale for neoadjuvant immunotherapy, with several pros and cons:
- Pros:
- Early treatment of micrometastatic disease
- Good patient performance status before surgery
- Enhanced immune response
- Tumor downsizing (earlier/less invasive/more organ-sparing surgery)
- Cons:
- Loss of surgical window: systemic toxicity postponing surgery, surgical safety, risk of progression during systemic treatment
- Lack of predictive biomarkers
- Complex design of clinical trials
The evidence from selected neoadjuvant immunotherapy trials is as follows:
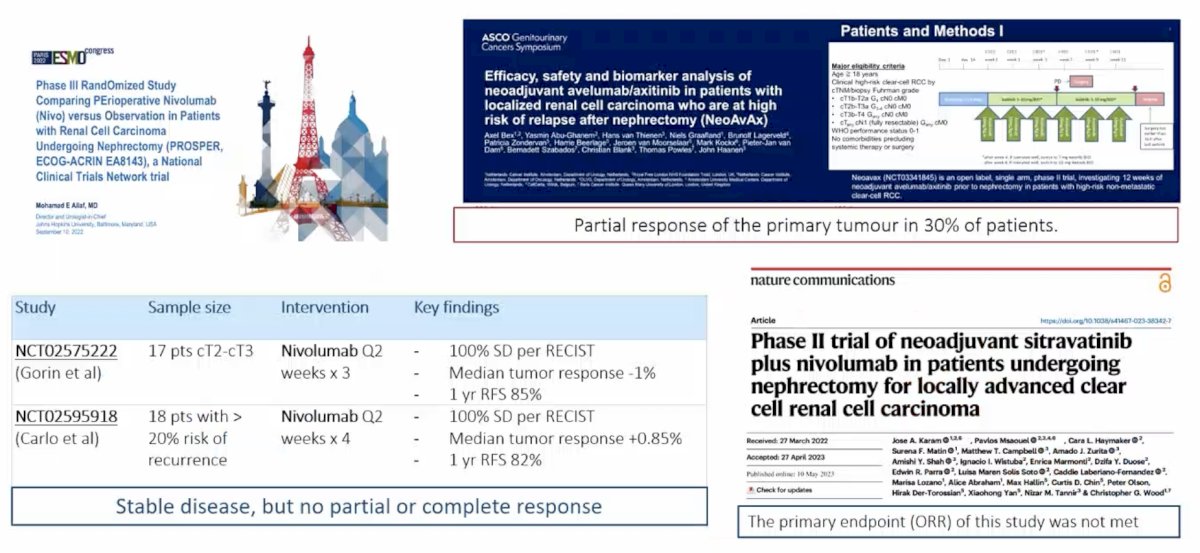
With regards to having the courage to guide the future, Dr. Campi emphasized that we need to improve risk-stratification (RCC biology and artificial intelligence), explore new biomarkers, align patient empowerment and individualized value-oriented care, and consider new trial designs and real work evidence. For new trial design, based on experience from other solid tumors (melanoma, breast, lung, colorectal), several central themes emerge: (1) Treatment de-escalation, (2) Neoadjuvant response-driven selection of candidates for adjuvant therapy, (3) Simple practice changing trials that are feasible across clinical settings and geographical areas, including low resource regions, to ensure equity and diversity in clinical research, and (4) The primary endpoints of the trials should be overall survival and quality of life improvement. For real world database studies, components of quality are important for fitness of purpose, data provenance, and transparency in methods and limitations.
Dr. Campi notes that renal cell cancers are different and the patients are different, thus we have to weigh the positives (impact on DFS and OS in one study, KEYNOTE-564), and the potential negatives:
- Conflicting evidence
- Unknown applicability of trial results to real-life populations
- Paucity of data on non-clear cell RCC
- Suboptimal outcome prediction, thus potential unnecessary treatment
- Adverse events and financial toxicity
- Drug availability and costs
- Patient preferences
- Challenging scenarios: M1 NED and recurrences after immunotherapy
Indeed, not all KEYNOTE-564 eligible patients are equal:
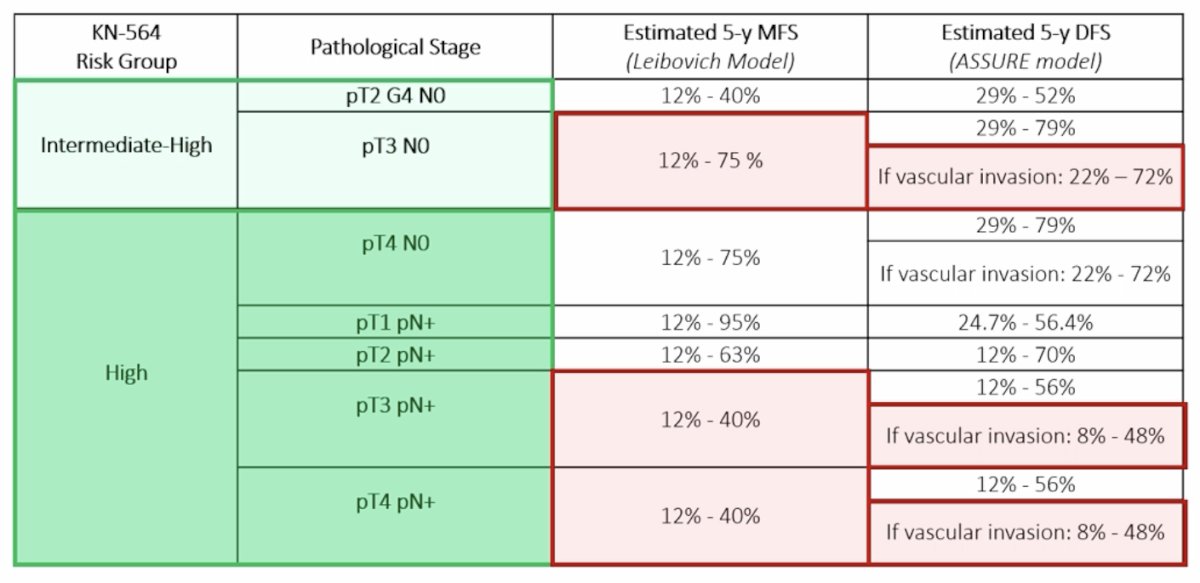
Moreover, the toxicity from immunotherapy can be serious, with a risk of short and long-term specific toxicity and the need for specific expertise to manage these toxicities. Thus, patients who might benefit the most from adjuvant pembrolizumab are those whose probability of RCC recurrence outweighs their probability of other-cause mortality over a reasonable timeframe after surgery.
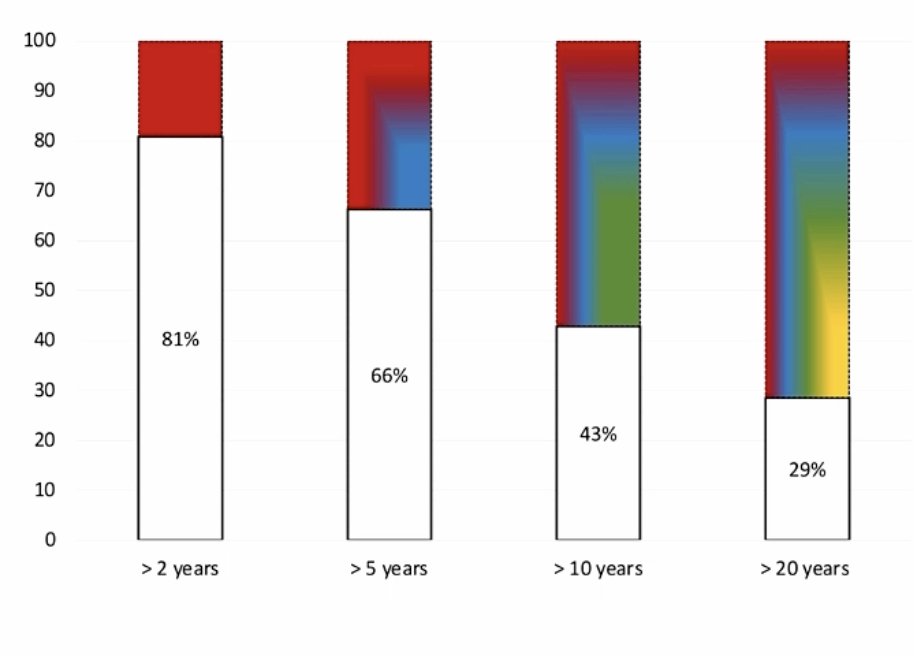
Recently, Dr. Campi and his group applied a risk-adapted approach to patient selection for adjuvant pembrolizumab.2 In this study, they stratified the patients using the risk-adapted model proposed by Stewart-Merrill et al. whereby stopping follow-up is warranted when the estimated risk of other cause mortality outweighs the estimated risk of RCC recurrence. They then explored the proportion of patients whose follow-up could theoretically be stopped at 2, 5, 10, or 20 years, for whom “eligibility” for adjuvant immunotherapy might be more controversial. Overall, 419/1,745 (24%) of patients with clear cell RCC met the KEYNOTE-564 criteria. The proportion of patients “not eligible” for adjuvant pembrolizumab would have been:
- 81% for recommended follow-up of >2 years
- 66% for recommended follow-up of >5 years
- 43% for recommended follow-up of >10 years
- 29% for recommended follow-up of >20 years
Dr. Campi concluded his presentation by discussing the future of immunotherapy in localized kidney cancer with the following conclusions:
- Immunotherapy has a future in localized RCC, yet the forecast is still nuanced
- Adjuvant pembrolizumab is (will be) a standard of care, but the available evidence is conflicting, prompting caution and critical thinking
- Neoadjuvant immunotherapy trials may offer opportunities to change perspective
- Patient selection is key and should be pursued by clinicians and healthcare systems
- The GU oncology community should design the pathway toward the future by leveraging a shared perspective and multidisciplinary collaboration
Presented by: Riccardo Campi, MD, PhD, FEBU, Careggi Hospital, University of Florence, Florence, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th – April 8th, 2024
References:
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.
- Campi R, Pecoraro A, Roussel E, et al. Could a risk-adapted approach support shared decision-making regarding eligibility for adjuvant pembrolizumab for patients with clear cell renal cell carcinoma at high risk of recurrence? A multicentre cohort study. Eur Urol Oncol. 2023 Nov 7 [Epub ahead of print].


