(UroToday.com) The 2024 European Association of Urology (EAU) annual congress held in Paris, France between April 5th and 8th was host to a plenary session addressing imaging-related controversies for the staging of genitourinary cancers. Professors Fred Witjes, Alison Birtle, and James Catto participated in a rapid-fire debate discussing the optimal treatment, from both an oncologic and quality-of-life (QoL) perspective, for young healthy patients with muscle-invasive bladder cancer (MIBC).
Professor Witjes presented the case of a 63-year-old previously healthy male (BMI: 22.4 kg/m2) who presented with a chief complaint of gross hematuria. Cystoscopy demonstrated the presence of a solitary tumor on the right-side wall, not involving the ipsilateral ureteral orifice. Staging CT demonstrated evidence of likely cT2N0M0 disease. A radical TURBT revealed muscle involvement. Deep and random biopsy sampling were all negative. Based on the clinical, radiologic, and pathologic data, Professor Witjes noted that both radical cystectomy and trimodality therapy (TMT) are options in this setting. This is supported by the recently published EAU 2024 guidelines, as summarized below:
Next, Professor Birtle made the case for TMT in this setting. Over the past decade, there has been strong evidence from numerous retrospective studies that oncologic outcomes are non-inferior for well-selected TMT patients, compared to patients undergoing radical cystectomy.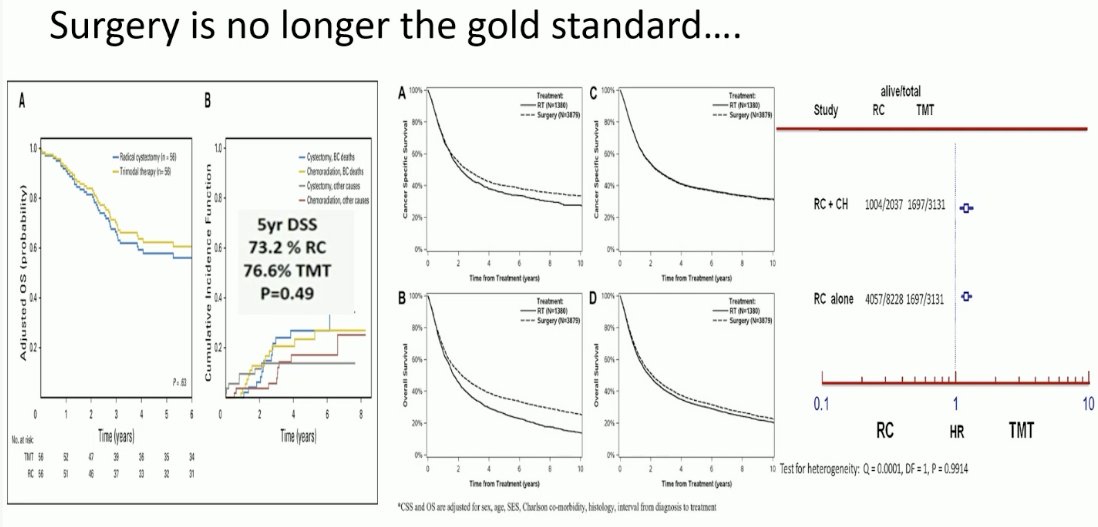
This has been most recently demonstrated by Professor Alexandre Zlotta and colleagues who demonstrated in a large, multicenter (Toronto, USC, MGH) cohort study that TMT in well-selected patients meeting pre-defined criteria was associated with favorable oncologic outcomes, compared to the historic reference standard of radical cystectomy.1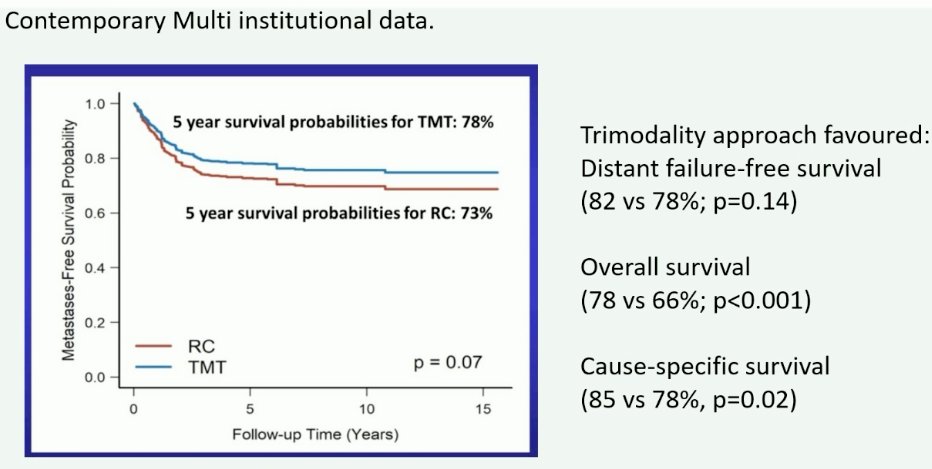
Why are we still debating this topic? There is zero Level 1 evidence in favor of one modality versus the other, and more recent data even suggests that bladder preservation may be superior. However, ultimately, the decision remains all about patient choice and clinical equipoise persists in this setting. While a randomized clinical trial of one versus the other would be ideal, evidence to date is that recruitment/randomization to such a trial remains challenging. The SPARE trial failed to accrue.2 Additionally, retrospective studies relying on multi-center series and population-based datasets have shown mixed results that are likely biased by numerous confounders.
Currently, in the UK, national guidelines advise that patients with MIBC see both a surgeon and a medical/radiation oncologist prior to making a treatment decision.
Current contraindications to bladder preservation include the following:
- Absolute: prior pelvic radiation
- Relative:
- Poor bladder function
- Inflammatory bowel disease
- Prognostic factors
- Tumor size
- Number of tumors
- CIS
- Hydronephrosis
From a quality-of-life perspective, data from the SPARE trial has demonstrated that although there are no overall differences in quality-of-life outcomes, there are meaningful differences in body image and sexual function favoring TMT.2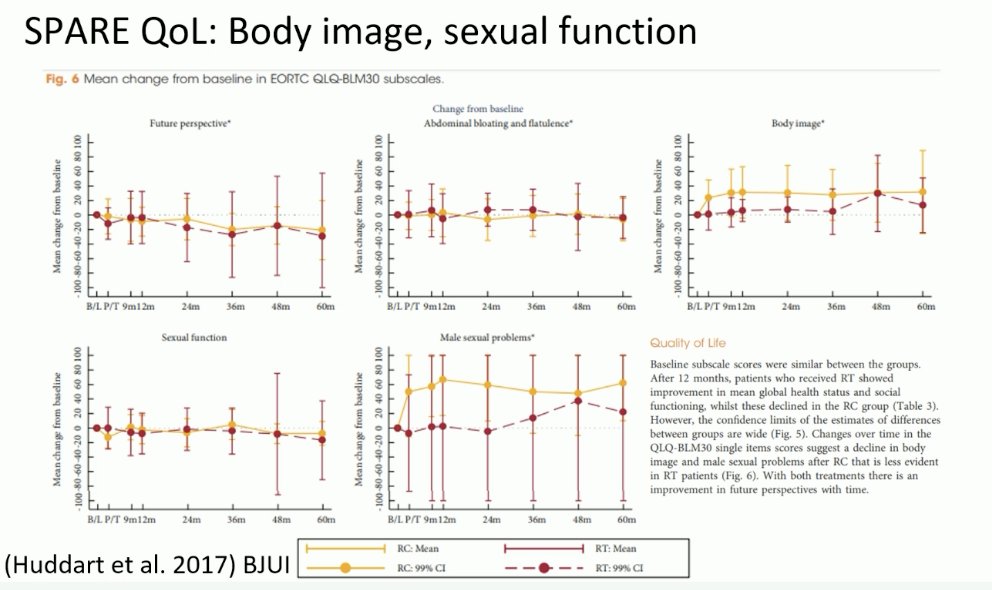
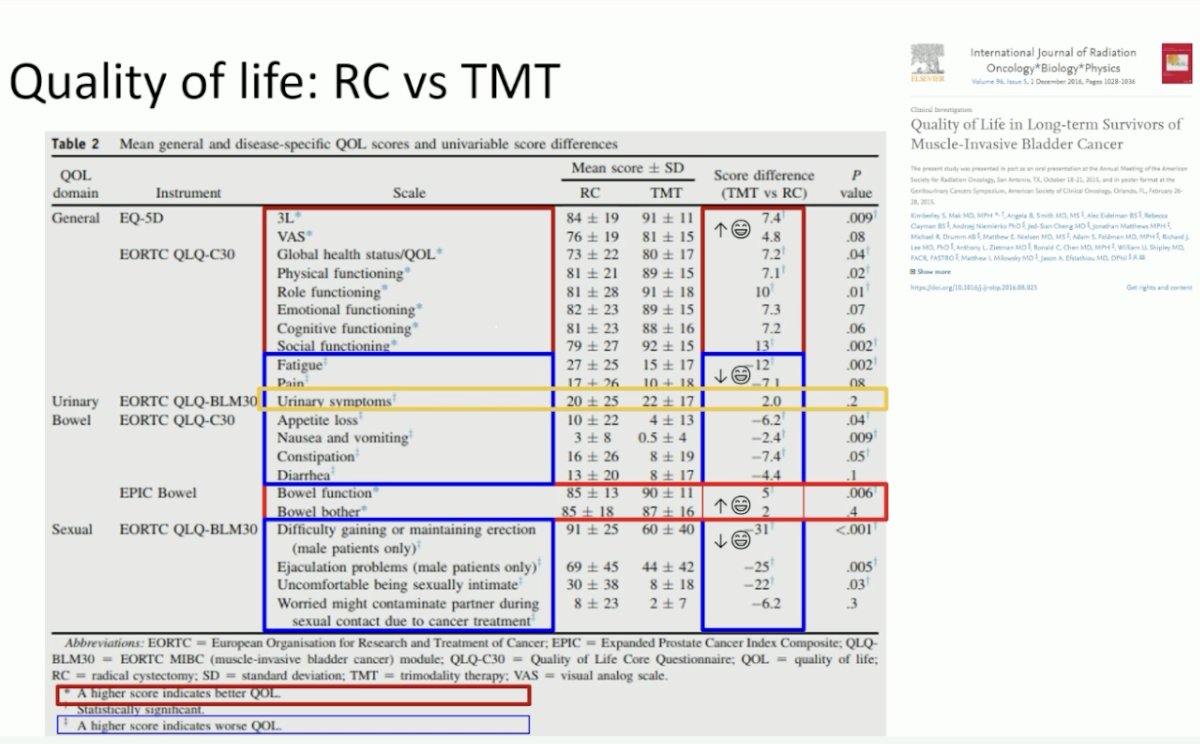
Additional long-term toxicity and quality of life data confirm that bladder preservation outperforms radical cystectomy in this setting. Late pelvic grade 3+ toxicity from RTOG and BC2001 trials appears to be in the 1–6% range. Compared to radical cystectomy, TMT is associated with:
- Modestly higher general quality of life
- Similar urinary scores
- Modestly higher bowel function
- Markedly better sexual quality of life
- Better informed decision-making
- Less concerns about appearance
- Less life interference from cancer or cancer treatment
What about the financial aspect of this decision-making process? While data published by Williams et al. in 2019 suggests that TMT is more expensive than radical cystectomy and that this disparity widens over time, Dr. Birtle noted that this study did not account for the robotic cystectomy component that is increasingly performed and for the costs of neoadjuvant chemotherapy prior to radical cystectomy.3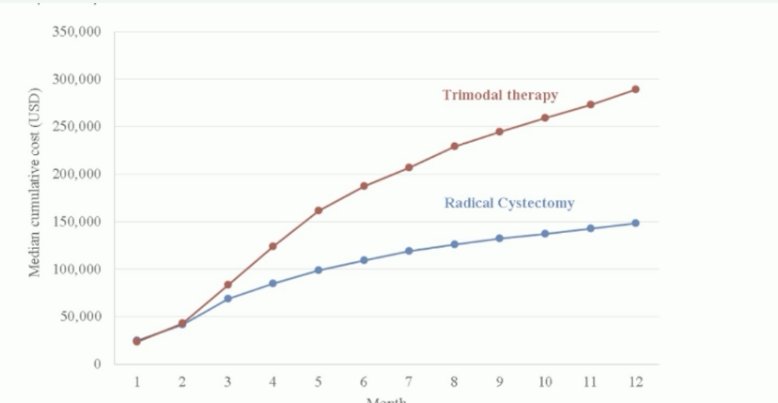
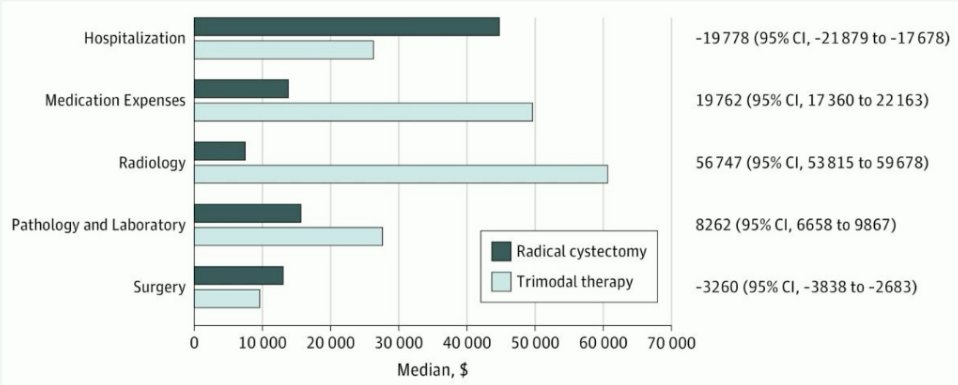
What about salvage cystectomy? Current series indicate that 5–10% of patients treated with TMT subsequently undergo salvage cystectomy. Importantly, such patients appear to have similar cancer-specific survivals compared to those who undergo primary cystectomy and the associated morbidity is acceptable.
What is the current state of radical cystectomy counseling and utilization in the UK? Most radical cystectomies are performed for muscle-invasive disease. Respondents with a delayed diagnosis were more likely to have a radical cystectomy. Notably, nearly three quarters (74%) of people who had a radical cystectomy said that no other treatment options were discussed with them to retain their bladder.
She concluded her presentation with this quote from Andrew Winterbottom, founder of the Fight Bladder Cancer Foundation: “It is crucial that everyone affected by bladder cancer – patients, carers, families and friends – have a reliable place to come to for support, information, and advice.”
Next, Professor Catto argued in favor of radical cystectomy for MIBC patients. While there is clearly a role for TMT, there are disease-, bladder-, and patient-specific factors that may preclude TMT:
- Disease
- Squamous cell carcinoma
- Adenocarcinoma (urachal and non-urachal)
- Sarcoma
- Widespread CIS
- Diverticular tumor
- Multifocal disease
- Bladder
- Storage problems: low compliance/volume
- Upper tract obstruction
- Patient
- Prior pelvic radiotherapy (e.g., for prostate cancer, testis cancer, cervical cancer)
- Inflammatory bowel disease or small bowel pathology
- Hip replacements
Although large, well-conduced retrospective series have demonstrated the non-inferiority of TMT, compared to radical cystectomy, the major caveat here is that included patients were well-selected, meeting strict inclusion criteria. In the study by Zlotta et al., all patients were required to have had solitary tumors <7 cm in size, no or unilateral hydronephrosis, and no extensive or multifocal CIS. These cases accounted for only 29% of all radical cystectomies performed during the study period at the contributing institutions, which indicates that 71% of patients either refused or were not eligible for TMT.1 In Professor Catto’s experience, the majority of patients in his clinical practice are not TMT eligible, with 53% of patients in Sheffield, UK harboring ≥cT3 disease and in the UK, overall, 43% have ≥cT3 disease at cystectomy.4 Challenges with patient recruitment to trials of TMT is exemplified by the SPARE trial, whereby 62% of the 796 patients screened were ineligible for TMT.2
He did emphasize however that there is clinical equipoise and a role for TMT among patients meeting the following criteria:
- Disease
- Muscle-invasive urothelial carcinoma of the bladder
- Possible N+ disease
- Variant urothelial histology
- Bladder
- Normal function. Minor bother. Good capacity.
- No or mild hydronephrosis
- Patient
- Good performance status (to tolerate either treatment)
- No contraindications
But is there a role for combining both modalities? Such a paradigm already exists for rectal, esophageal, and pancreatic cancers.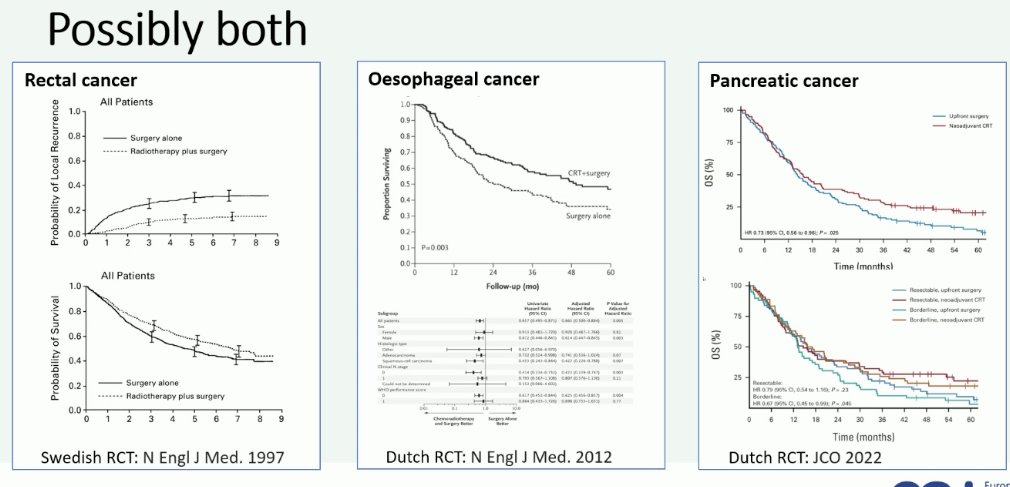
In bladder cancer, there appears to be a subset of patients that might benefit from local treatment pre-radical cystectomy:5
- Stage T3–4
- Nodes N1–2
- Histology: Non-urothelial carcinoma and some urothelial carcinoma variants
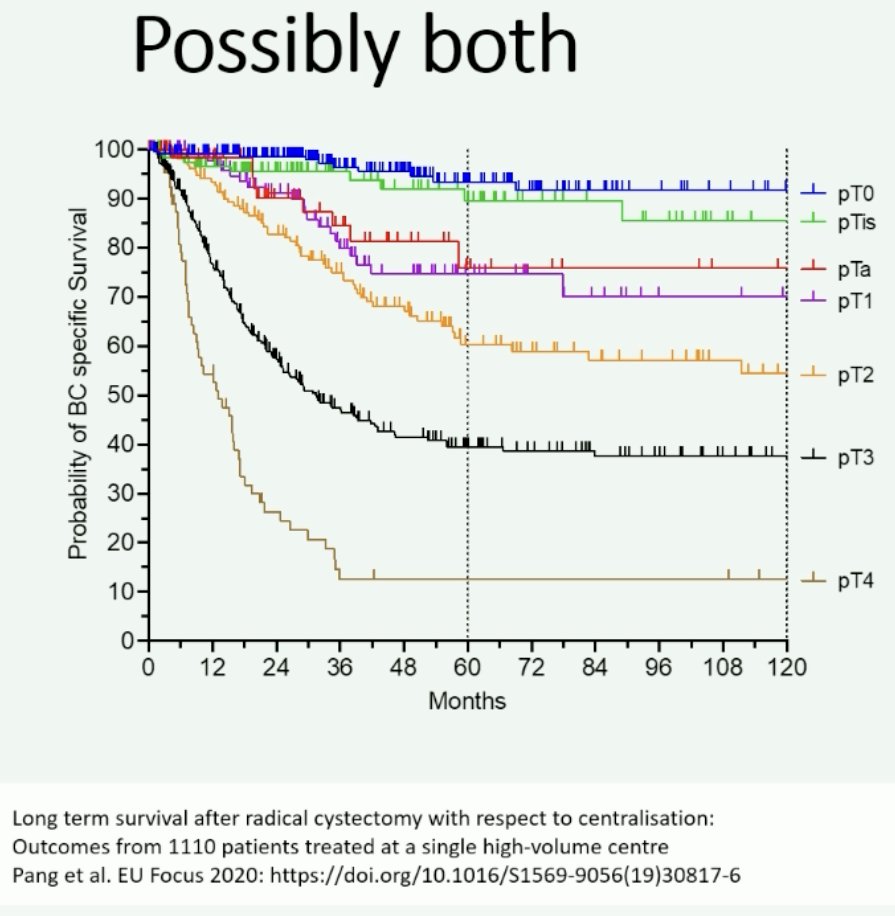
A German trial presented at ESMO 2022 evaluated ‘triplet’ therapy combination with radiotherapy + immunotherapy (nivolumab) prior to radical cystectomy. Of the 33 enrolled patients, 87% completed all three treatment. 39% of patients were found to have pT0 disease at radical cystectomy. The incidence of grade 3–4 adverse events was 35%.
Presented by:
- Professor Fred Witjes, MD, PhD, Department of Urology, University Hospital, Nijmegen, The Netherlands
- Professor Alison Birtle, MBBS, MRCP, FRCR, MD, Consultant Oncologist and Honorary Clinical Professor at Lancashire Teaching Hospitals NHS Foundation Trust, Preston, UK
- Professor James Catto, MBChB, PhD, FRCS, Professor of Urologic Surgery at the University of Sheffield, Sheffield, United Kingdom
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:- Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: A multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023 Jun;24(6): 669-681.
- Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE - a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017;120(5): 639-650.
- Williams SB, Shan Y, Ray-Zack MD, et al. Comparison of Costs of Radical Cystectomy vs Trimodal Therapy for Patients With Localized Muscle-Invasive Bladder Cancer. JAMA Surg. 2019;154(8): e191629.
- Jeffries ER, Cresswell J, McGrath JS, et al. Open radical cystectomy in England: the current standard of care - analysis of the British Association of Urological Surgeons (BAUS) cystectomy audit and Hospital Episodes Statistics (HES) data. BJU Int. 2018;121(6): 880-885.


