PSMA is a transmembrane protein that is often highly expressed by prostate cancer cells and not express by most normal tissues (exception = salivary glands). Expression correlates to advanced disease. It is less expressed by liver metastases.

PSMA-PET Imaging is clearly more sensitive than other imaging modalities. While there are some false positives and false negatives, it is still a very sensitive tool.
Beyond diagnostics, there was subsequent interest in leveraging the specificity of PSMA for therapeutic approaches. One of the approaches gaining the most interest is so-called “theranostics” – in prostate cancer, this approach leverages molecularly targeting cancer cells using prostate-specific membrane antigen (PSMA)-targeting radioligands. As PSMA is highly expressed in prostate cancer and mCRPC lesions, the combination of PSMA-617 with the beta-emitter lutetium allows for the targeted delivery of ß-particle radiation to PSMA-expressing cells and the surrounding microenvironment
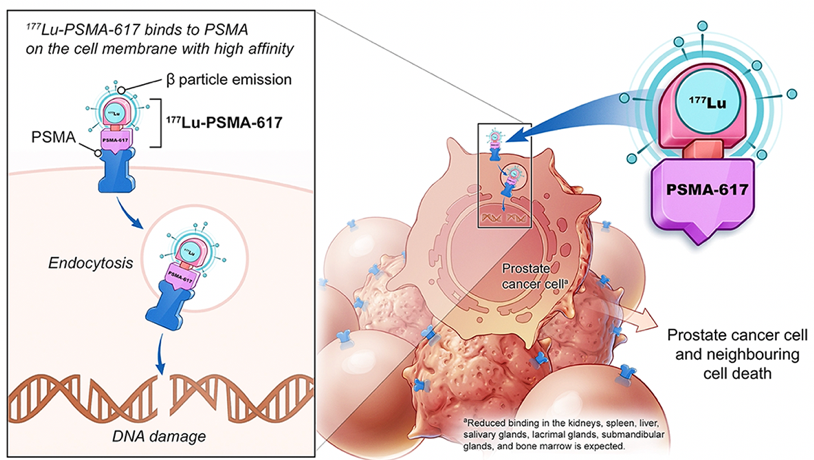
The VISION study,1 presented earlier this year at ASCO, is a randomized phase 3 study that assessed the addition of Lutetium-PSMA to standard of care therapy in men with (often heavily pretreated) mCRPC.
The study design is below:
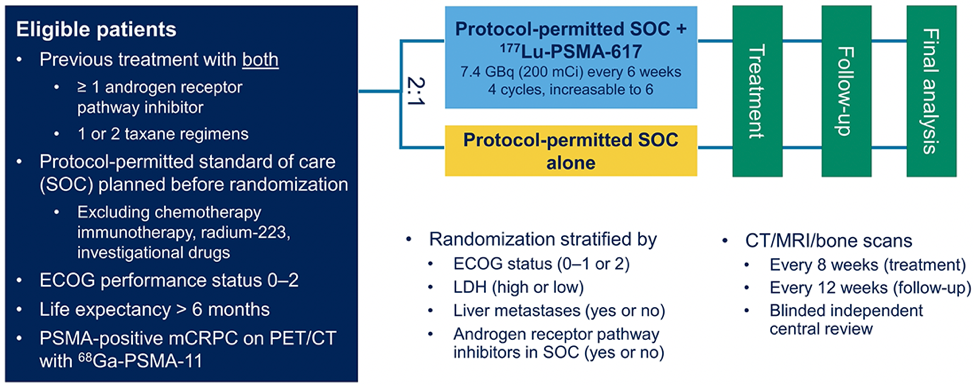
Of 1003 screened patients, 869 (86.6%) met PSMA criteria (PET avid lesion, no visible PET dark CT lesions).
Over a median study follow-up of 20.9 month (as of a data cut-off of 27 January 2021), treatment with 177Lu-PSMA-617 + SOC significantly improved overall survival by a median of 4.0 months (median overall survival (OS), 15.3 vs 11.3 months; HR, 0.62 [95% CI: 0.52, 0.74]; p < 0.001, one-sided), compared to SOC alone, in the overall cohort of all randomized patients (n=831).
Importantly, it also demonstrated an impressive objective response rate – with 9% (vs. 0% of controls) have complete response and 41.8% (vs. 3.1% of controls) have partial response.
This is obviously an important development and will likely be incorporated into guidelines.
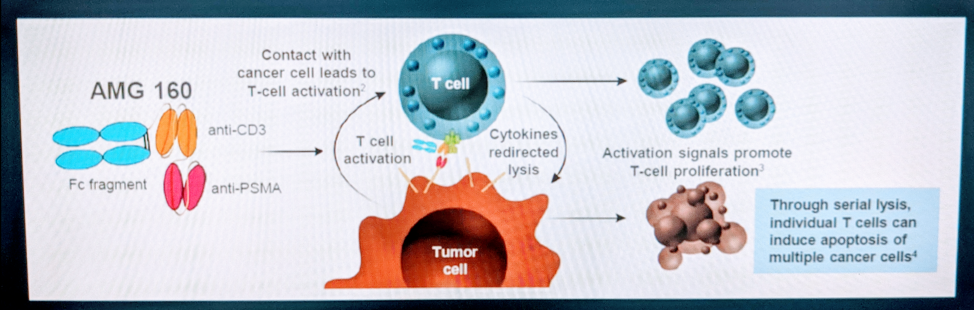
He did then discuss other methods of using PSMA in a therapeutic approach. One such approach is the BITE (Bispecific T-cell Engager approach). In this approach, an anti-PSMA and anti-CD3 antibody are linked together – and by doing so, it helps bring T-cells and tumor cells in contact, thereby helping promote immune system recognition of tumor cells.
This is demonstrated below:
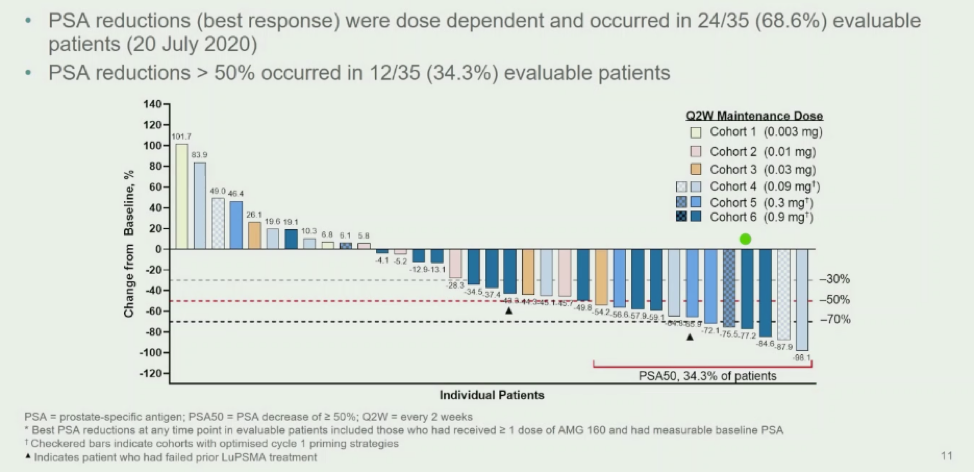
AMG160 is one such PSMA BITE agent and, in an ESMO 2021 abstract, was demonstrated to have a 68.8% PSA response – and over 34.3% had a >50% PSA reduction. Full results are found here:
Another BITE agent is HPN424, whose results were presented at ASCO 2021 (by Dr. De Bono). These also had a significant PSA response with therapy.
However, there are still numerous unanswered questions regarding PSMA in prostate cancer therapeutics.
- What is the optimal dose of PSMA-lutetium
- Can we use alpha emitters instead?
- Should we discontinue PSMA-Lutetium if PET shows no more uptake?
- Can we / Should we re treat patients if they have recurrence?
- Can it be used in an earlier setting?
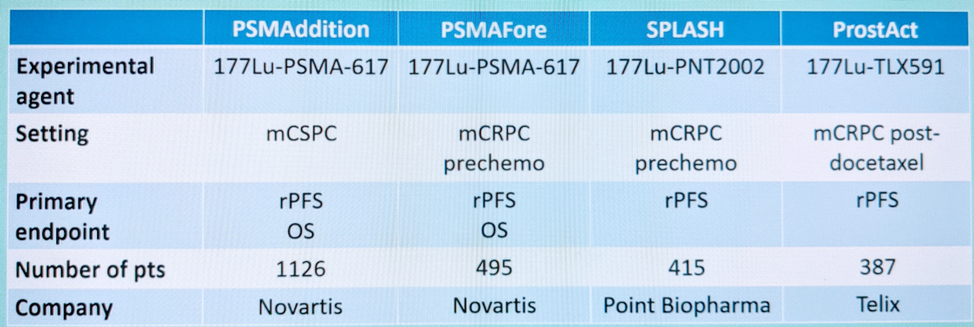
The upcoming phase 3 trials in earlier disease states are shown below:
At the end, his concluding remarks are:
1. PSMA = excellent target
2. PET-PSMA is the most sensitive current imaging
3. PSMA targeting works:
VISION – OS improved with PSMA-lutetium
Optimal use of PSMA-Lutetium is yet to be defined
BITES are promising
4. Patients treated with PSM a targeting still die – so we need to better understand resistance to treatment. Perhaps earlier use may provide more meaningful benefits.
Presented by: Karim Fizazi, MD, Ph.D., is a medical oncologist, Head of the Department of Cancer Medicine at the Institute Gustave Roussy (IGR), Villejuif, France and a Professor in Oncology at the University of Paris.
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:


