(UroToday.com) The European Association of Urology (EAU) 2021 Virtual Annual Meeting included a joint session of the EAU and the Canadian Urological Association and a presentation by Dr. Christian La Fougere discussing prostate radioligand therapy and its use earlier in the disease process. Dr. La Fougere notes that the theranostic key-lock principle includes a target (ie. membrane-antigens, PSMA), a ligand (ie. small molecules, PSMA-617), a linker, and a chelator (ie. PET-reporting unit, 68Ga, 64Cu, 18F; or therapeutic unit, 90Y, 177Lu, 213Bi, 225Ac):

Radioligand therapy takes advantage of an anti-proliferative effect in that internal radiotherapy is administered by molecules (ie. 177Lu-PSMA, median range 0.67 mm) with limited radiation to adjacent normal cells.
Dr. La Fougere highlights that early clinic work was out of Germany, whereby in 2017 a multicenter study published outcomes of 145 patients with mCRPC treatment with 177Lu-PSMA-617.1 A total of 248 therapy cycles were performed in 145 patients with a median follow-up of 16 weeks (range, 2-30 weeks). The overall biochemical response rate was 45% after all therapy cycles, whereas 40% of patients had already responded after a single cycle. Elevated alkaline phosphatase and the presence of visceral metastases were negative predictors and the total number of therapy cycles positive predictors of biochemical response.
To highlight evidence from prospective clinical trials, Dr. La Fougere discussed two recent impactful clinical trials, VISION2 and TheraP.3 The VISION trial is an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Patients in VISION were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. The trial schema for VISION is as follows:

There were two primary endpoints for VISION: radiographic progression-free survival using PCWG3 criteria by independent central review and overall survival. Key secondary endpoints included objective response rate (ORR; RECIST v1.1), disease control rate (DCR), and time to first symptomatic skeletal event (SSE) as well as other secondary endpoints including safety and tolerability, biomarkers including PSA, and health-related quality of life and pain. Among 1,179 screened patients, the VISION trial enrolled 831 patients, including 551 patients allocated to 177Lu-PSMA-617 + standard of care, and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved overall survival by a median of 4.0 months (median OS, 15.3 vs 11.3 months; HR, 0.62, 95% CI 0.52, 0.74; p < 0.001, one-sided), compared to standard of care alone:
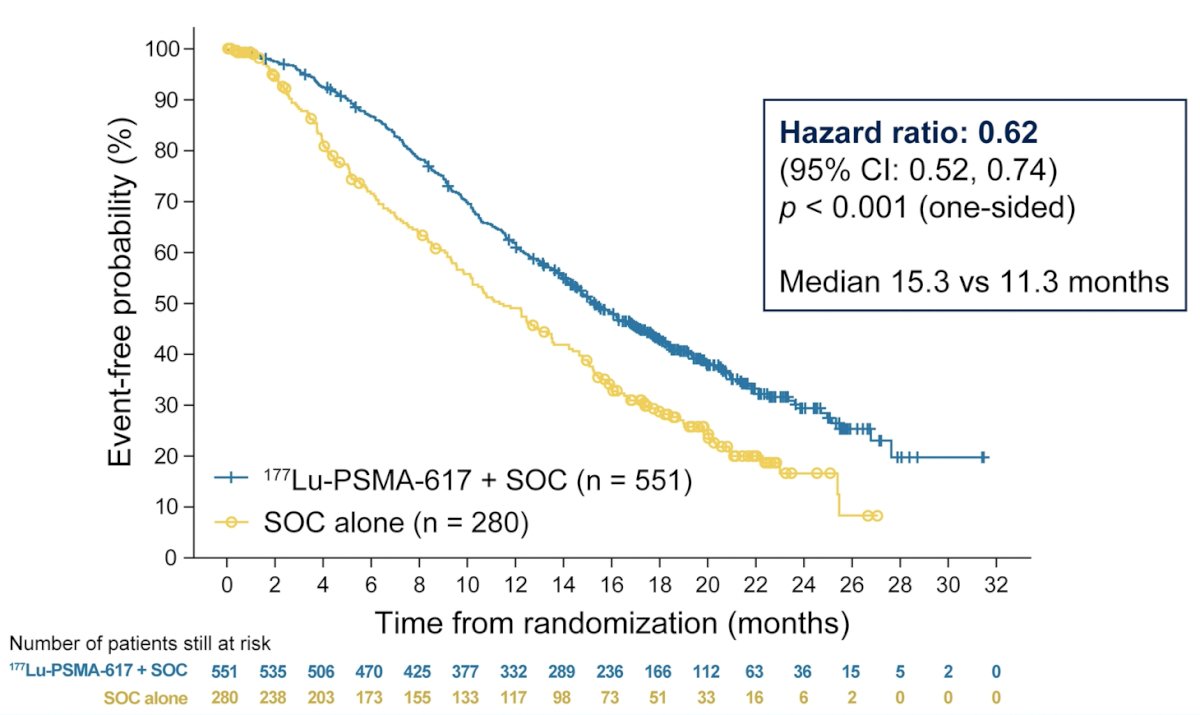
The second, alternate primary endpoint showed that treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR, 0.40, 99.2% CI: 0.29, 0.57; p < 0.001, one-sided).
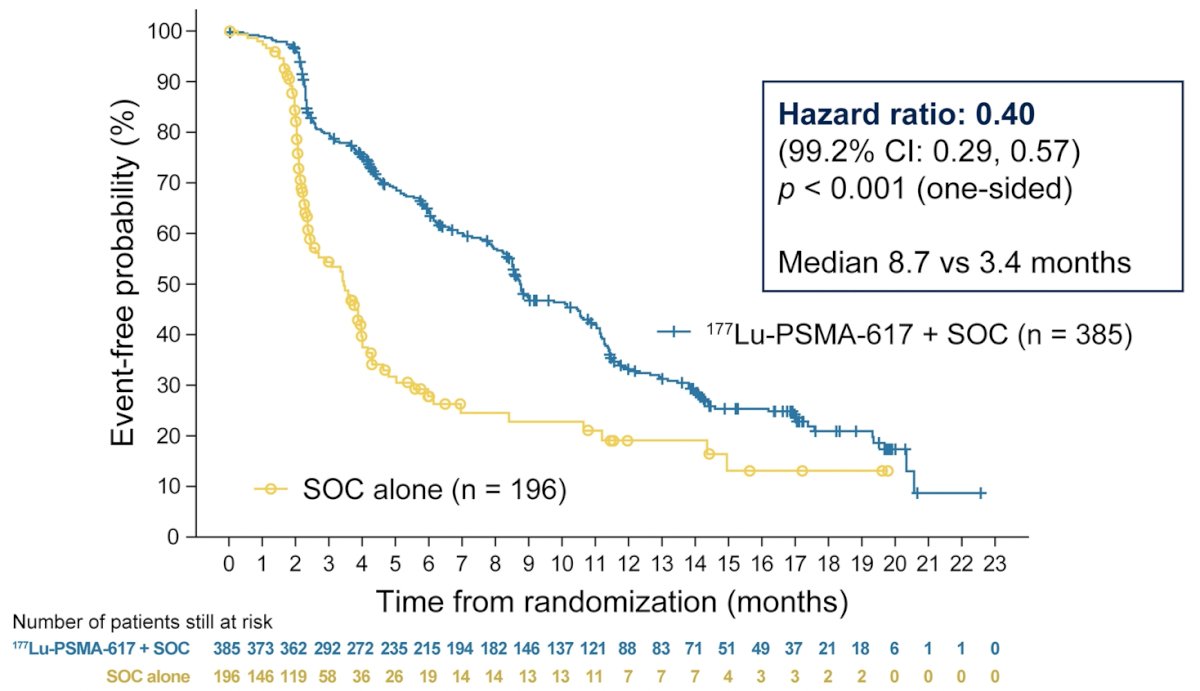
In addition to the primary endpoints, the addition of 177Lu-PSMA-617 to standard of care statistically significantly improved all key secondary endpoints, including ICR-determined ORR (29.8% vs 1.7%), ICR-determined DCR (89.0% vs 66.7%) and time to first SSE (median time, 11.5 vs 6.8 months; HR, 0.50). Further, PSA responses (whether defined as a 50% decrease or an 80% decrease) were significantly more common among those treated with 177Lu-PSMA-617 + standard of care.
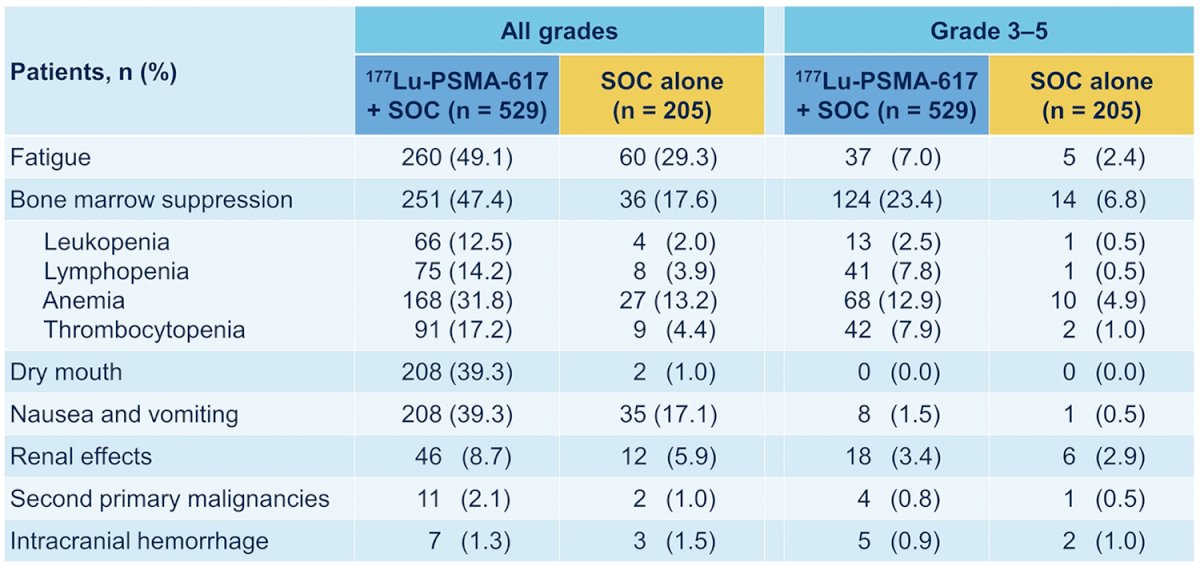
While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% vs 3.9%), overall therapy was well tolerated. In terms of specific adverse events, treatment with 177Lu-PSMA-617 + standard of care was associated with increased rates of bone marrow suppression, xerostomia, and nausea and vomiting:
Dr. La Fougere then discussed the TheraP trial which is an ANZUP/PCFA sponsored trial that began in 2016. TheraP enrolled patients with mCRPC who had previously received docetaxel and were eligible to receive cabazitaxel. Patients were required to have progressive disease with a rising PSA with absolute PSA of 20 ng/mL or higher. All patients underwent both Ga-68-PSMA-PET/CT and F-18-FDG-PET/CT prior to randomization. To be eligible for inclusion, patients must have had a high avidity lesion on PSMA PET/CT (SUV max >20 at any site) with measurable disease with SUV max of 10 or greater. Further, there could be no sites of disease which were FDG positive but PSMA negative. The trial schema for TheraP is as follows:
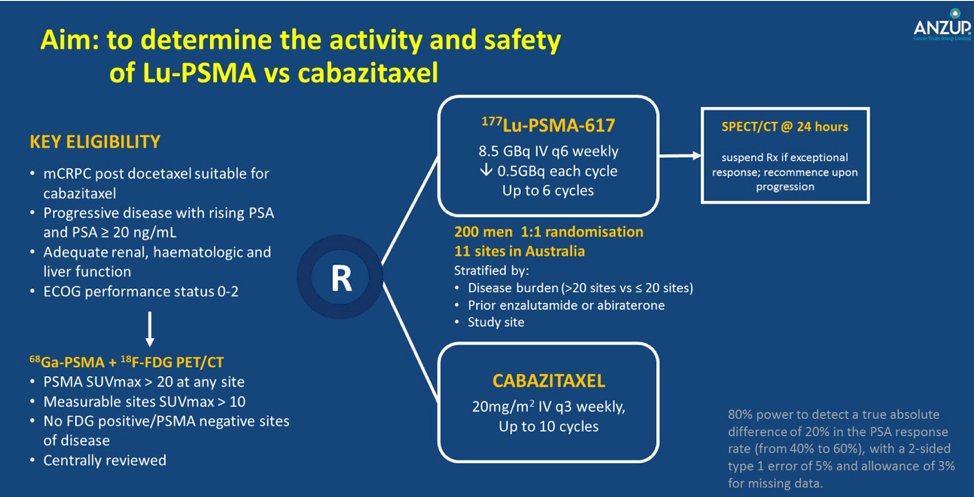
Among 200 men at 11 sites in Australia who were eligible, randomization was performed in a 1:1 fashion to 177Lu-PSMA-617 or cabazitaxel. Randomization was stratified according to disease burden, prior use of enzalutamide or abiraterone, and study site. The primary study outcome was PSA response, operationalized looking at a response of at least 50% from baseline.
Compared to those receiving cabazitaxel (37%, 95% confidence interval 27 to 46%), responses were significantly higher among those who received Lu-PSMA (66%, 95% CI 56 to 75%) with an absolute difference of 29% (95% CI 16 to 42%, p<0.0001). Over a median follow-up of 18.4 months, PFS was significantly longer in those assigned Lu-PSMA rather than cabazitaxel (rates at 1 year 19% [95%CI 12-27%] vs 3% [1-9%], HR 0.63, 95%CI 0.46-0.86; p = 0.003) based on 173 events:
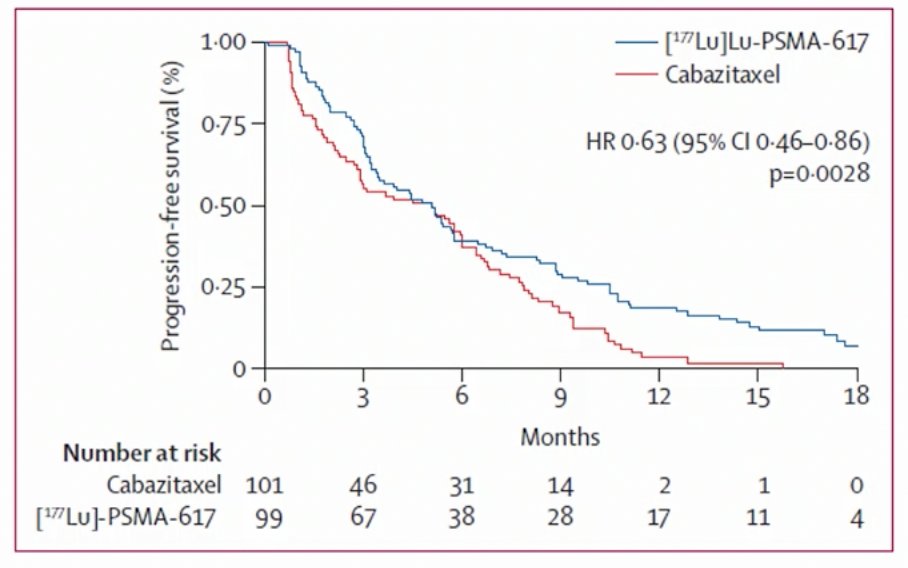
Among men with measurable disease (n=78), objective response rates were significantly greater in the Lu-PSMA arm (49% vs 24%, RR 2.1, 95% CI 1.1-4.1; p = 0.019). Similarly, among those with pain at baseline (n=90), pain responses occurred in 60% in the Lu-PSMA arm vs 43% for cabazitaxel (RR 1.42, 95% CI 0.84-4.48; p = 0.10). Dr. La Fougere noted that the main limitation of TheraP is that we are still awaiting overall survival data.
Dr. La Fougere then summarized the existing evidence for using radioligand therapy in mCRPC:
- It is well tolerated with limited side effects, of which the most common is bone marrow suppression
- Data in extensively pretreated mCRPC suggests that radioligand therapy rather than standard of care is superior (and probably is for second line chemotherapy, although overall survival data from TheraP is missing)
- Adapted and repeated radioligand therapy cycles is feasible (ie. 6 cycles), however there is clinical experience in different centers of patients tolerating up to 10 or more cycles
- PET-based (PSMA & FDG) therapy stratification seems to be the most beneficial, and is likely a key issue for early use of radioligand therapy (ie. prior to first line chemotherapy)
Dr. La Fougere concluded by noting that there are many steps needed for an earlier use:
- Comparison of radioligand therapy versus first line chemotherapy after ARPI: there is a high demand from patients and self-help groups
- This is a relatively new treatment approach in aggressive tumor entities: we need close interdisciplinary interactions between nuclear medicine, urology, oncology, and radiation oncology
- The combination of radioligand therapy with additional treatment approaches (ie. PARP inhibitors or immunotherapy) remains to be determined
- There are unanswered questions, including what is the discrepancy between PSA decline and appearance of new PSMA-positive lesions during radioligand therapy
Presented by: Christian La Fougere, MD, University Hospital Tubingen, Tubingen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Rhabar K, Ahmadzadehfar H, Kratochwil C, et al. Germany multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017 Jan;58(1):85-90.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Jun 23 [Epub ahead of print].
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.


