Figure 1- Prostate cancer treatment 2000:

Figure 2 – Prostate cancer treatment 2020:

There has also been significant progress in the use and research of biomarkers in the realm of prostate cancer. It is important to understand three different and critical biomarker definitions :
- Prognostic biomarker - which is one that indicates an increased or decreased likelihood of a future clinical event, disease recurrence, or progression in an identified population with testing done at baseline.
- Predictive biomarker which is used to identify individuals who are more likely than similar individuals without the biomarker to experience a favorable or unfavorable effect from exposure to a medical product, and it is also tested at baseline
- Therapeutic response biomarker which is used to identify a pharmacological or physiological response from the treatment. This may or may not extrapolate to the clinical benefit
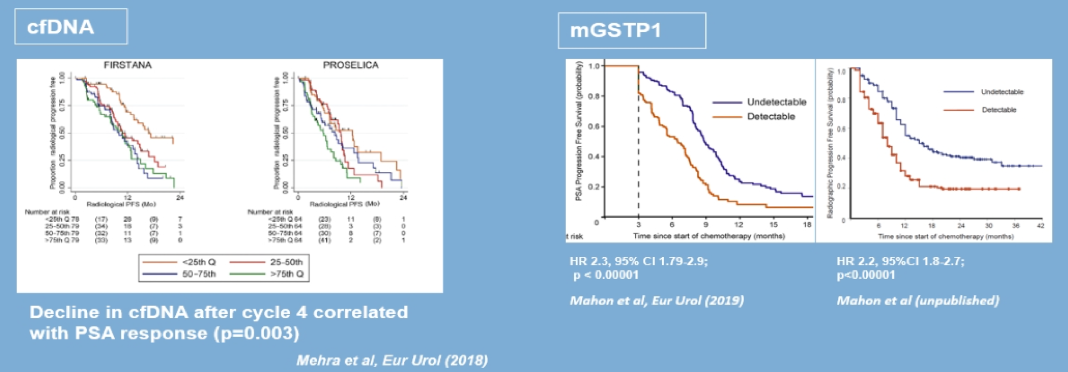
Taxanes, which have been shown to cause a 30% or more declining prostate-specific antigen (PSA) at three months in metastatic castrate-resistant prostate cancer patients treated with it, have also been shown to correlate with therapeutic response biomarker effects. For example, a decline in Circulating free DNA after the 4th cycle of taxane was correlated with PSA response (Figure 3).
Figure 3 – Taxanes-Therapeutic response biomarkers (progression-free survival):
This has also been shown in the use of androgen receptor pathway inhibitors (abiraterone and enzalutamide) in the treatment of metastatic castrate-resistant prostate cancer, when compared to clinically localized prostate cancer, with different genomic aberrations (Figure 4), and a clear correlation to various predictive biomarkers (Figure 5).
Figure 4 – CRPC vs. Localized prostate cancer:
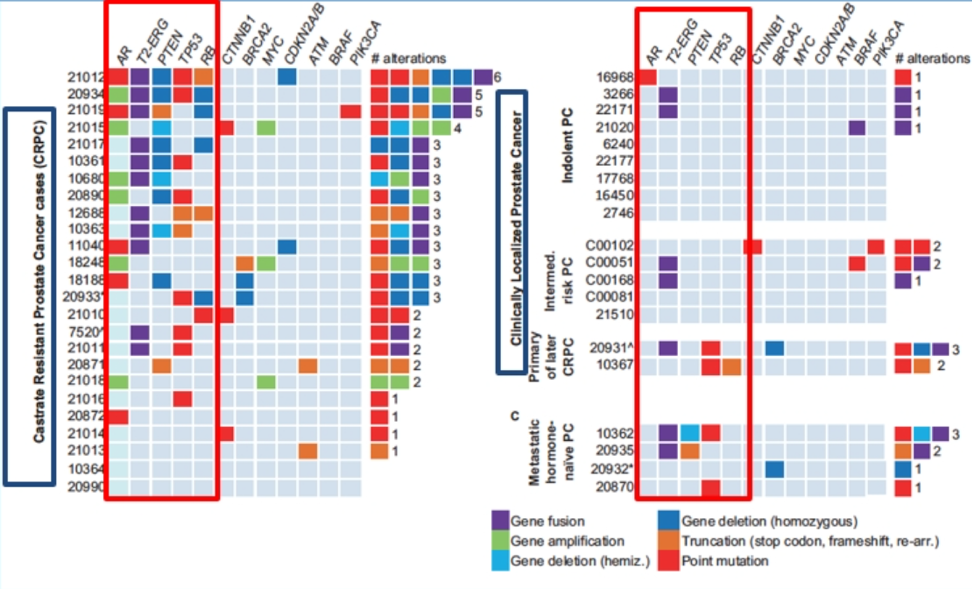
Figure 5 — ctDNA genomic changes and androgen receptor pathway inhibitors – predictive biomarkers:

Lastly, Dr. Horvath discussed the novel treatment of the PARP inhibitors (olaparib) which have shown to cause a significant response in patients with homologous recombination repair (HRR) defects, with BRCA 1 and 2, and ATM shown to be the HRR defects which are most likely to respond to Olaparib (Figure 6).
Figure 6 – HRR defects most likely to respond to treatment with olaparib:

It is important that we continue to implement the critical step of moving the biomarkers from the lab to the clinic. We need to make sure that the biomarker has analytical validity; in other words, does the test reliably measured the biomarker. We also need clinical efficacy to show that the test reliably separates patients into two or more groups. Lastly, we need to show clinical utility, demonstrating that the use of the test leads to improved clinical outcomes.
Lisa Horvath, MBBS, FRACP, PhD, concluded her talk by stating that currently, we can use circulating tumor cell (CTC) enumeration, and HRR defects for PARP inhibitors, to predict response to systemic therapy in patients with metastatic castrate-resistant prostate cancer.
There is currently ongoing research in tissue genomics for clinical trials of targeted therapies. Researchers are also exploring the role of circulating tumor DNA (ctDNA), circulating free DNA (cfDNA), Mgstp1 (ANZUP study), and immune and metabolic biomarkers. All these will require prospective clinical utility studies before implementation in the real world practice can occur.
Presented by: Lisa Horvath, MBBS, FRACP, PhD, Director of Medical Oncology, Director of Research, Conjoint Chair of Medical Oncology (Genitourinary Cancers), Chris O'Brien Lifehouse, Sydney, Australia
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020/


