To date, there are generally three different options for intravesical treatment:
- Single-dose chemotherapy
- BCG or chemotherapy induction therapy and maintenance
- Device assisted
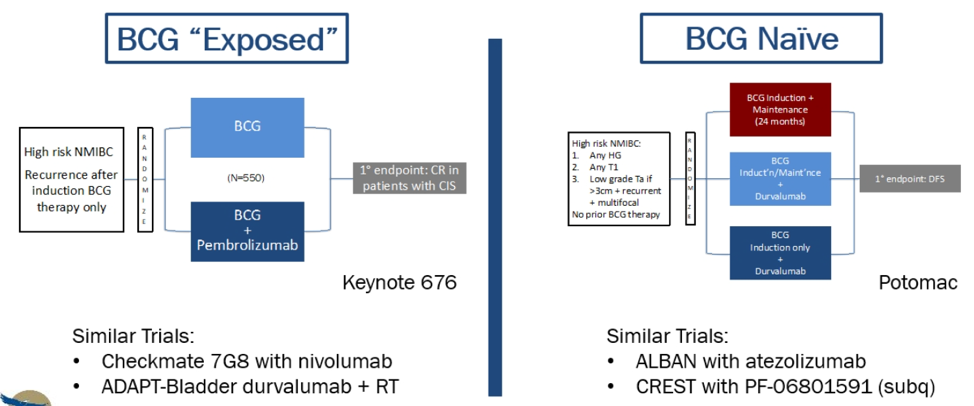
Dr. Black divides NMIBC into three categories (Figure 1):
- BCG naïve
- BCG “exposed.”
- BCG unresponsive
Figure 1 – BCG categories:

S1602 is a randomized trial evaluating the influence of BCG strain differences and T cell priming for BCG-naïve high-grade NMIBC.
In the next part of the discussion, Dr. Black Elaborated on five novel intravesical therapies (figure 2).
Figure 2- Novel intravesical therapy:

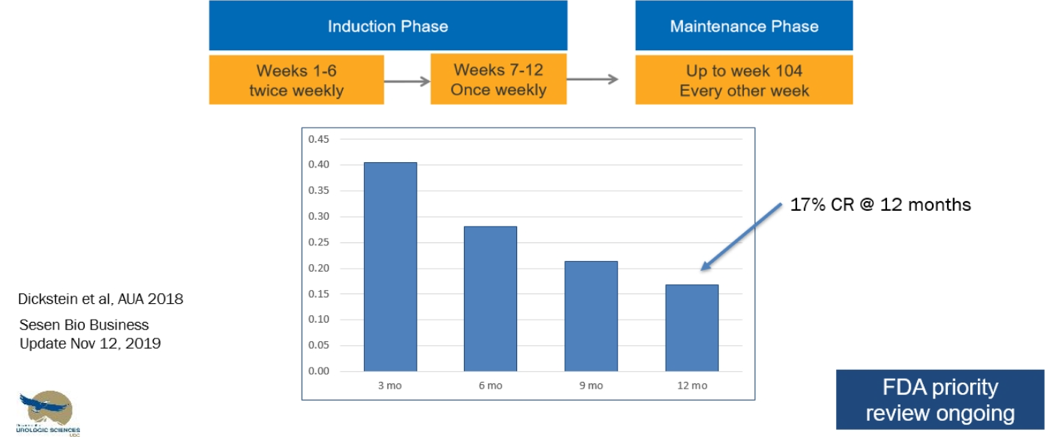
Oportuzumab monatox is an antibody-drug conjugate. The antibody binds cell surface EPCAM, which is seen on epithelial cells, and especially cancer cells. The payload delivered is a pseudomonas Toxin A that goes into the cell and leads to cell death. It is given intravesically twice weekly over six weeks, followed by a once-weekly dosage for another six weeks, and then every other week for up to 2 years. The VISTA trial, a phase 3 single-arm trial, demonstrated a 17% complete response rate at 12 months in BCG unresponsive patients with oportuzamb monatox. (Figure 3).
Figure 3 – VISTA trial results:
Nadofaragene firadenovec is a form of gene therapy of adenovirus carrying IFN-a2b + SYN3 as an excipient. The complete response rate at 3, 6,9, and 12 months have been shown to be 53%, 41%, 35%, and 24%, respectively, and it is also currently undergoing FDA priority review.
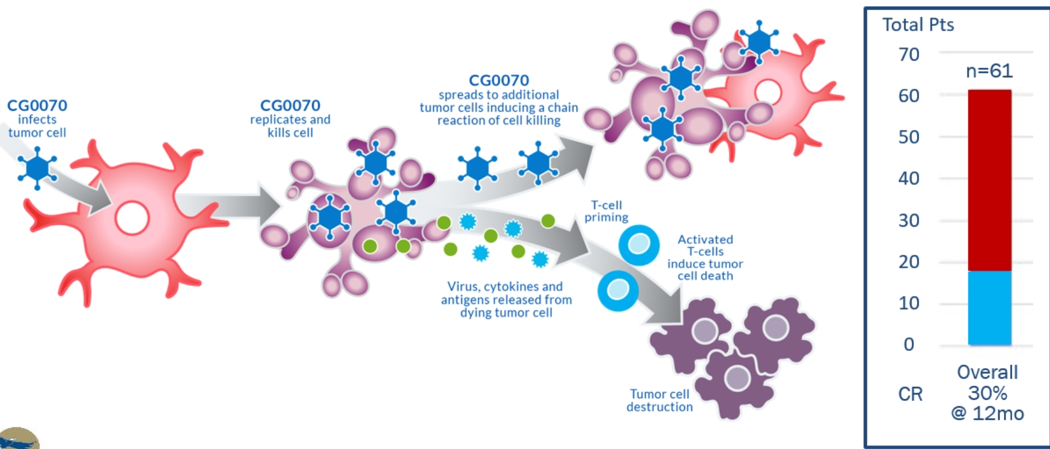
CG0070 Oncolytic virus is another form of intravesical therapy. This virus infects the tumor cell, replicates inside it, and kills it. It spreads to additional tumor cells inducing a chain reaction of cell killing. The virus and the associated cytokines and antigens are released from the dying tumor cell, causing T-cell priming, and these activated T cells induce tumor cell death (figure 5)
Figure 5 – Mechanism of CG00070 Oncolytic virus:
The next novel intravesical therapy that was discussed was the ALT-803, which is the interleukin 15 superagonist complex with a complete response rate of 82% at three months.
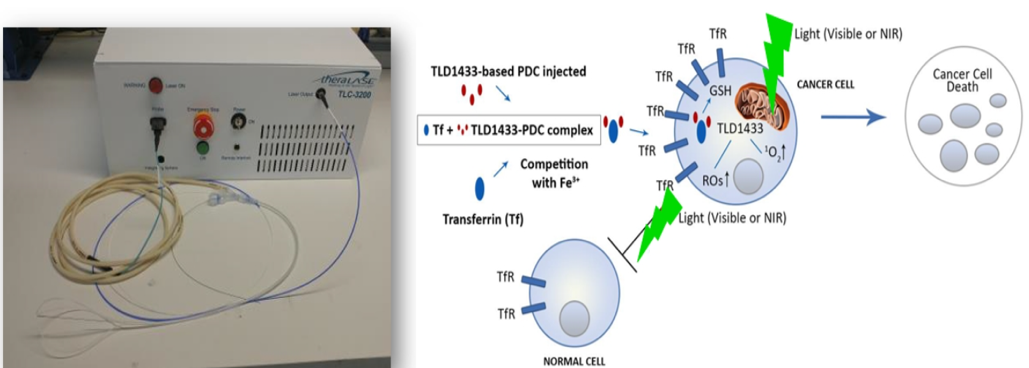
The fifth drug discussed was the TLD1433 photodynamic therapy which consists of an intravesical injection of TLD1433 based PDC, which is activated by a light source from the cystoscopy. A small 6-patient phase 1 safety trial was completed for dosage and safety, and currently, there is a large phase II trial that is underway for BCG unresponsive NMIBC patients. The significant advantage of this therapy is that this is only a one-time treatment that can be repeated after six months (figure 6).
Figure 6 – TLD1433 photodynamic therapy:
Next, Dr. Black discussed the novel systemic therapies, which include the anti-PD-L1 and FGFR inhibitors.
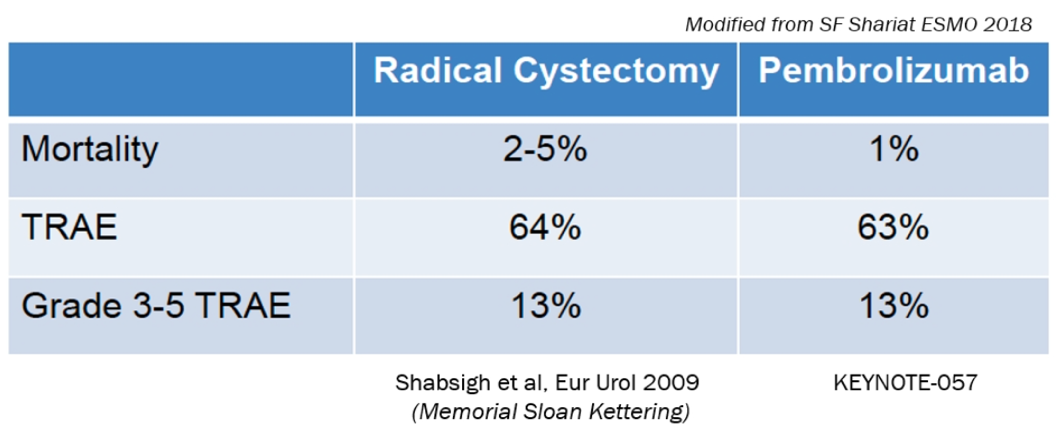
Two trials have assessed the effect of pembrolizumab (Keynote 057) and Atezolizumab (SWOG S1605) in NMIBC patients with CIS that was unresponsive to BCG (Figure 7). Pembrolizumab has been FDA approved for this indication. Pembrolizumab has been compared to radical cystectomy, demonstrating very similar results (Figure 8). Another significant advantage is that patients that recurred after pembrolizumab treatment remained eligible for radical cystectomy.
Figure 7 – PD-L inhibitors for BCG unresponsive CIS:

Figure 8 - Comparison of pembrolizumab to radical cystectomy in NMIBC patients:
There are currently other ongoing BCG-unresponsive trials (Figure 9), and the trend is to try and administer PD-L1 inhibitors even earlier for NMIBC (Figure 10).
Figure 9 - ongoing BCG-unresponsive trials:

Figure 10 – PD(L)-1 inhibitors for earlier NMIBC:
Presented by: Peter Black, MD, FACS, FRCSC, University of British Columbia, Vancouver, Canada
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the Virtual 2020 EAU Annual Meeting #EAU20, July 17-19, 2020
Related Content:
A Debate on The Management of BCG Unresponsive - Cystectomy Ineligible Bladder Cancer Patient : Pembrolizumab Vs. Nadofaragene Firadenovec - Arjun Balar & Peter Black


