(UroToday.com) As part of the “Game-changing Session 2” plenary presentation at the European Association of Urology (EAU) Virtual Annual Meeting, Dr. Neal Shore presented results of the phase III trial of nadofaregene firadenovec in patients with high-grade, bacillus calmette-guerin or BCG-unresponsive non-muscle invasive bladder cancer on behalf of Dr. Boorjian, Dr. Dinney, and the Society of Urologic Oncology (SUO) Clinical Trials Consortium (SUO CTC).
Most patients newly diagnosed with bladder cancer have non-muscle invasive disease (NMIBC). For patients with intermediate or high-risk NMIBC and those with carcinoma in situ (CIS), adjuvant treatment with BCG is guideline-recommended on the basis of proven benefits in disease recurrence. While BCG is efficacious, many patients eventually develop BCG-unresponsive disease and are at risk for tumor recurrence and progression. For many years, there have been very limited options for these patients. Radical cystectomy has remained the gold standard though numerous approaches including intravesical and systemic therapies have been investigated. One of the alternatives to radical cystectomy in patients with BCG-unresponsive NMIBC is nadofaragene firadenovec, a novel intravesical gene-mediated therapy that delivers the human IFNα2b gene resulting in sustained IFNα2b expression, and provided durable responses in a previous Phase 2 trial.
The authors, through the auspices of the SUO CTC, conducted a multicenter, open-label Phase 3 study investigating nadofaragene for high-grade NMIBC (carcinoma in situ [CIS ± Ta/T1], or PD [Ta/T1 alone]) unresponsive to BCG (NCT02773849).
Nadofaragene (3X1011 vp/mL [75 mL]) was administered intravesically once every 3 months for up 4 doses in the initial 12 months. Patients without evidence of high-grade disease at month 12 were offered continued treatment at the investigator’s discretion. While the primary outcome was complete response rate (CR) among patients with CIS which has previously been reported, key secondary end points included rate and durability of high-grade-recurrence-free (HGRF) survival as well as safety.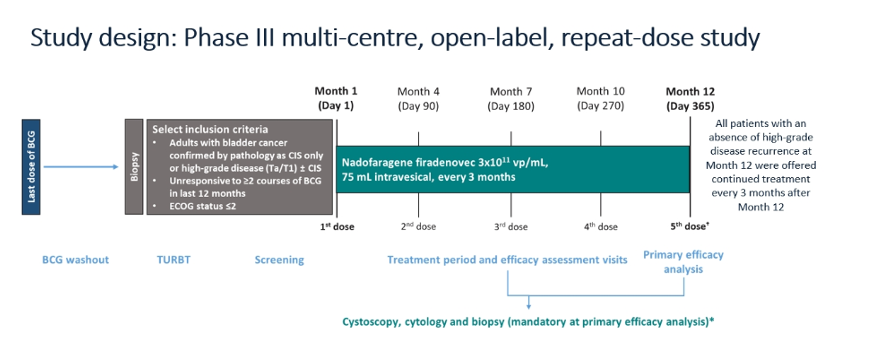
Patients were stratified into those with CIS (n=107) and those with papillary only disease (n=50). In general, characteristics were similar between these groups, though BCG-refractory, rather than relapsed disease, was more common in the papillary subgroup. In keeping with an expected bladder cancer population, mean patient age was over 70 years and the vast majority were male.
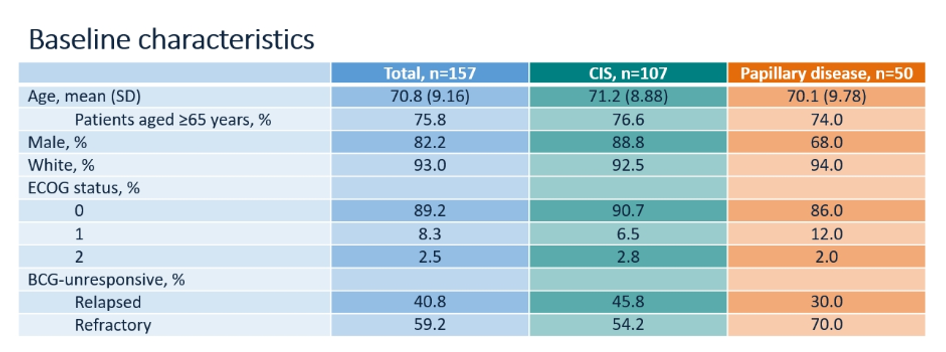
72% of included patients had received two or three courses of BCG: 68.3% of those with CI and 80% of those with papillary disease had received two or three courses of BCG.
In the primary efficacy analysis, the authors examined complete response rate in the subset of patients with CIS. This demonstrated a 12-month complete response of 53.4% (95% confidence interval 43.3 – 63.3%; n=55) among those patients in the CIS cohort. Interestingly, all complete responses occurred in the first 3 months. Complete response rates were somewhat higher in the papillary subgroup. On these basis of these data, the primary outcome was met.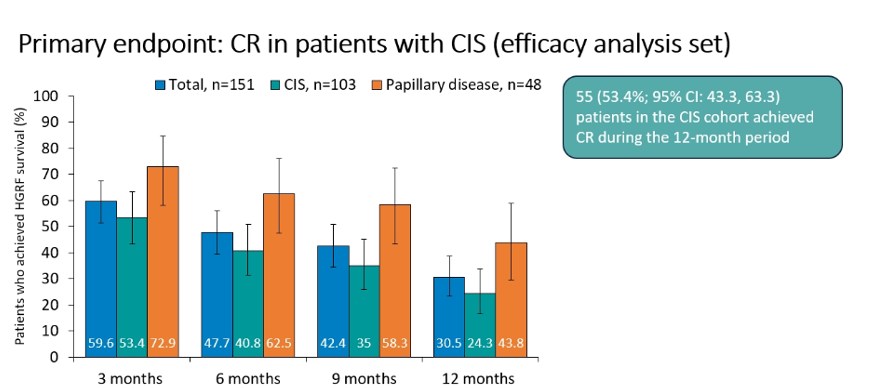
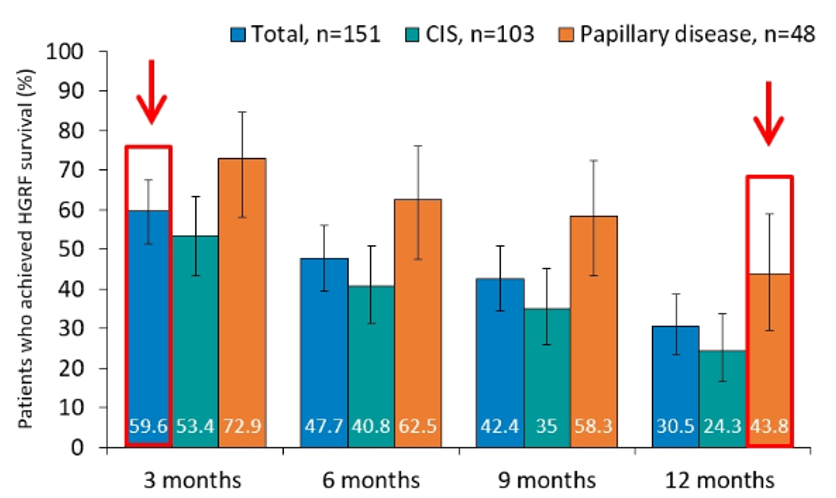
Key secondary outcomes also supported the role of nadofaregene firadenovec. Among the efficacy analysis set, high-grade recurrence free survival and durability were promising with 12-month recurrence free survival of 24.3% (95% confidence interval 16.4-33.7%) in the CIS cohort and 43.8% (95% confidence interval 29.5-58.8%) in the papillary cohort. In the absence of mandated biopsy, rates would have been somewhat higher. Among patients with papillary disease, median high-grade recurrence free survival was 12.4 months.
In addition to efficacy endpoints, the authors assessed the safety of nadofaregene firadenovec. Again, the authors stratified according to CIS and papillary only disease. While any treatment related adverse events were common (93%), serious treatment adverse events were uncommon (8.9%) with no grade 5 adverse events. Further, treatment related adverse events leading to discontinuation occurred in only 1.9% of patients. The majority of treatment related adverse events were instillation site discharge, fatigue, bladder spasm, urinary urgency, and hematuria.
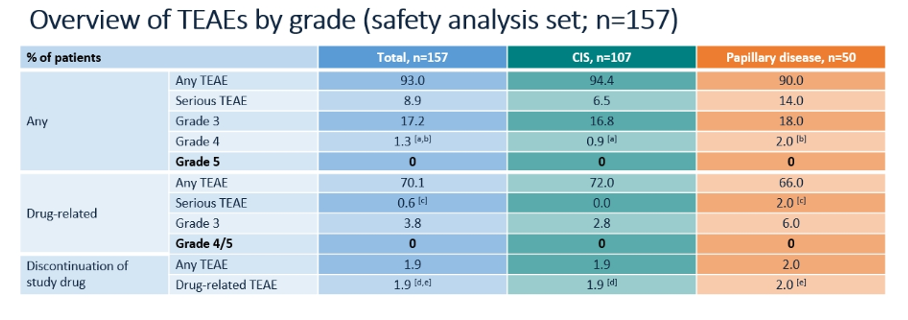
The authors conclude that nadofaregene firadenovec demonstrates clinical response in patients with BCG-unresponsive NMIBC for patients with both CIS and papillary disease. Further, it is well tolerated.
Presented by: Neal D. Shore, MD, FACS is the Medical Director for the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina.
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter at the 35th Annual EAU Congress, 2020 Virtual Program #EAU20, July 17-19, 2020.
Related Content:
Watch: Results of the SUO CTC Phase III Nadofaragene Firadenovec Trial for BCG Unresponsive NMIBC - Colin Dinney
Read: SUO 2019: The SUO-CTC Phase III Adstiladrin® Trial for BCG Unresponsive Non-Muscle Invasive Bladder Cancer
Watch: Setting the Stage for the BCG Unresponsive Population - Interview with Ashish Kamat


