For this study, bone scintigraphs were centralized and retrospectively analyzed for M1 patients randomized between October 1, 2008 and March 31, 2013 to the STAMPEDE control arm (A) or a docetaxel-containing arms (C and E).
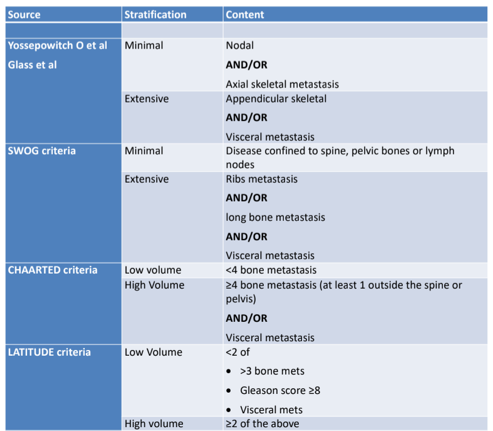
Metastatic disease burden was classified according to the CHAARTED volume definition3, as well as the Yossepowitch5 and LATITUDE6 trial definitions:
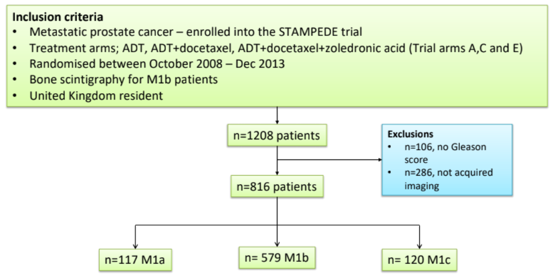
For this study, 1,208 patients were identified meeting the inclusion criteria, and 816 patients had incomplete clinical information for imaging centralization making up the study cohort. Metastatic status distribution included: M1a (n=117), M1b (n=579, 78%), M1c (n=120).
There was significant variation in percent prevalence in high and low volume STAMPEDE trial patients when stratified by alternative definitions:
- Yossepowitch: 23.4% low-volume; 76.6% high-volume
- Yossepowitch (with non-regional lymph nodes and bone metastasis in high-volume): 21.4% low-volume; 78.6% high-volume
- CHAARTED: 40.2% low-volume; 59.8% high-volume
- CHAARTED: (with non-regional lymph nodes and bone metastasis in high-volume): 35.8% low-volume; 64.2% high-volume
- LATITUDE: 44.9% low-volume; 55.1% high-volume
- LATITUDE (with non-regional lymph nodes and bone metastasis in high-volume): 42.2% low-volume; 57.8% high-volume
Presented by: Alex P. Hoyle, Christie NHS Foundation Trust, Manchester, United Kingdom
Co-Authors: Ali A, Douis H, Sydes M, James N, Clarke N
References:
1. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
2. Gravis G, Boher JM, Joly F, et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70(2):256-262.
3. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737-746.
4. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018 Jan 31 [Epub ahead of print].
5. Yossepowitch O, et al. The natural history of noncastrate metastatic prostate cancer after radical prostatectomy. Eur Urol 2007;51(4):940-948.
6. Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352-360.
Written by: Zachary Klaassen, MD, Urologic Oncology Fellow, University of Toronto, Princess Margaret Cancer Centre, twitter: @zklaassen_md at the 2018 European Association of Urology Meeting EAU18, 16-20 March, 2018 Copenhagen, Denmark
Read More:
Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy - STAMPEDE Trial
Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer - CHAARTED Trial
Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer - LATITUDE


