There has been significant stage migration partly due to new imaging modalities, including PSMA based PET scans, Choline PET, whole body MRI, FDG-PET imaging, C11 Choline radiotracers, and sodium fluoride PET.
Both the primary tumor and the metastasis can seed new metastasis; however, it is mainly done by the metastasis. The CRPC treatment options both in the post- and pre-docetaxel space since 2015 have evolved to include chemotherapy, abiraterone, enzalutamide, radium and Sipleucel. Currently, the optimal sequence of drugs is unknown. There are currently several trials investigating the sequence of drugs (Figure 2). ARV7 – the androgen receptor splice variant, which lacks the ligand binding domain, but remains constitutively active, is a new biomarker for androgen receptor (AR) targeting resistance. It may predict response to abiraterone and enzalutamide and is not associated with lack of response to docetaxel.
There are also several novel agents, approaches and multi-modality treatments that are being investigated. Novel agents focusing on Non-AR targets include PARP inhibitors (Olaparib), and radio-immunoconjugates. Furthermore, immunotherapy is being investigated with a combination of multiple immune agents. Currently investigated immunotherapy agents include Ipilimumab, Duvalumab, Tasquinimod, prostate cancer vaccine (PROSTVAC), and live attenuated double deleted Listeria immunotherapy. Most of these are currently in ongoing trials pending results. The addition of radiotherapy to immunotherapy is also being investigated. Radiotherapy induces cell death and immunogenic modulation may enhance the effect of immunotherapy. Lastly, Lutetium-177 labeled anti-prostate-specific-membrane antigen (PSMA) monoclonal antibody is also being assessed in mCRPC.
Development of metastasis is associated with increased morbidity and mortality. There is an increased rate of metastasis or death in men with non-metastatic CRPC ad PSA doubling time <8-10 months. Androgen signaling inhibitors such as apalutamide and enzalutamide may delay metastasis and improve radiologic progression free survival (PFS). Enzalutamide has already been shown to prolong PFS and survival in men with mCRPC. The recently published SPARTAN trial randomized non-metastatic CRPC patients to either apalutamide and androgen deprivation therapy (ADT) or placebo and ADT. A clear benefit was shown for the apalutamide arm with improved metastasis-free survival (MFS) (by over 2 years) in all patient subgroups analyzed (Figure 3). A clear benefit for apalutamide was also seen in median PFS, overall survival (OS), time to symptomatic progression, time to PSA progression, and PSA response rate. Overall apalutamide with ADT was well tolerated among the patients These impressive results led to FDA approval of apalutamide for non-metastatic CRPC patients on the 14th of February 2018.
The PROSPER trial randomizing non-metastatic CRPC patients to either placebo + ADT or enzalutamide +ADT showed very similar results in favor of the enzalutamide arm. The Enzalutamide was demonstrated to have improved MFS (Figure 4) by 71%. Enzalutamide also demonstrated improved median time to PSA progression, median time to first new antineoplastic therapy, and median OS, compared to placebo. Treatment with enzalutamide and ADT was generally well tolerated. There are additional similar ongoing trials assessing the effect of darolutamide with ADT (ARAMIS trial) in men with high risk non-metastatic CRPC. Figure 5 summarizes the place of different treatment options in the progression of prostate cancer.
In summary, AR remains a major target in mCRPC even after progression on abiraterone and / enzalutamide. Optimal sequencing of drugs has not been established yet. It is important to keep all the drug options available. Patients with short response duration to 1st line ADT might harbor AR resistance to AR-targeted agents. In these patients, it is important to measure ARV7. In the absence of visceral metastasis, it is preferable to administer early AR targeting agents. If visceral metastasis occurs, it might be better to administer chemotherapy. Lastly, it is critical to invest in research to improve personalized medicine.
Figure 1: Trials demonstrating survival advantage in metastatic castrate resistant prostate cancer:

Figure 2: Current available trials investigating the correct sequencing of drugs:
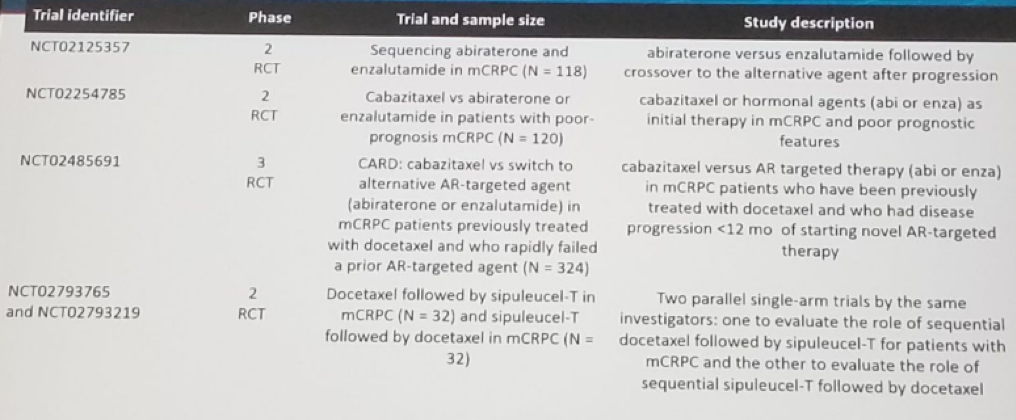
Figure 3: Metastasis free survival benefit for the Apalutamide arm in the SPARTAN trial:
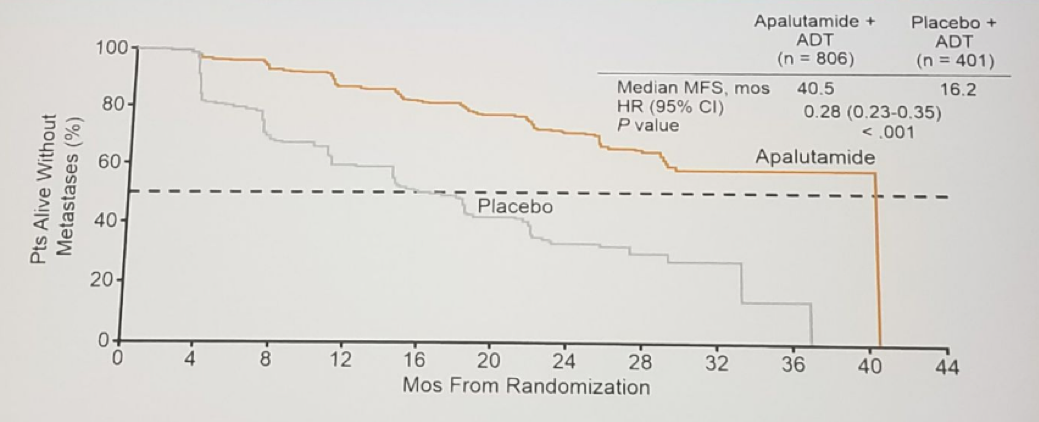
Figure 4: Metastasis free survival benefit for the enzalutamide arm in the PROSPER trial:
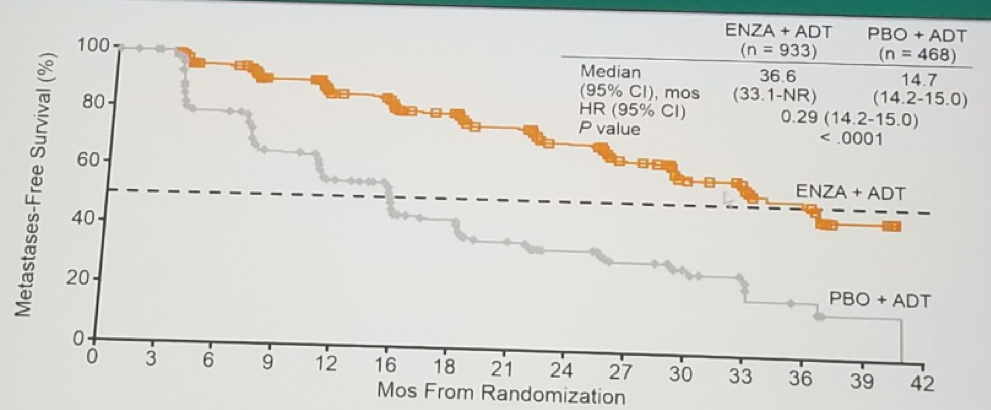
Figure 5: The role of different treatment options in the progression of prostate cancer:
Presented by: S. Osanto, Leiden, The Netherlands
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the 2018 European Association of Urology Meeting EAU18, 16-20 March 2018 Copenhagen, Denmark
Read More:
Radiographic Progression-Free Survival as a Clinically Meaningful End Point in Metastatic Castration-Resistant Prostate Cancer - the PREVAIL Randomized Clinical Trial
First Presentation - SPARTAN: A Study of Apalutamide (ARN-509) in Men with Non-Metastatic Castration-resistant Prostate Cancer


