(UroToday.com) The 2021 American Urological Association (AUA) Summer School session on Upper Tract Urothelial Carcinoma included a case-based discussion led by moderator Dr. Surena Matin who was joined by panelists Dr. Sima Porten and Dr. Vitaly Margulis. This case included a discussion of the role of adjuvant therapy. This patient was a 66-year-old female referred after undergoing a laparoscopic nephrectomy for a right renal mass. Pathology showed a pT4pN1R0 high-grade urothelial carcinoma with no ureteral tumors and a negative ureteral margin:

Considering the patient’s risk of metastatic recurrence and the risk of recurrence in the ureteral stump, Dr. Matin asked his panel what the next steps would be with the following options:
- Adjuvant chemotherapy
- Resection of the retained ureteral stump
- Surveillance only
Dr. Margulis states that it is always important to assess a patient’s risk of recurrence, which will help guide subsequent management. As such, the worry is less what is going to happen in the ureteral stump but what will happen from a systemic disease standpoint, which will drive treatment, and therefore she should be considered for adjuvant platinum-based chemotherapy.
There are several nomograms available for assessing risk of recurrence, specifically the following nomograms predicting 5-year survival:
- 2010 Karakiewicz nomogram: 72% accuracy
- 2012 Cha/Shariat nomogram: 76.8% accuracy
- 2013 Roupret nomogram: 80% accuracy
- 2014 Seisen nomogram: 81% accuracy
Additionally, the Krabbe nomogram1 (n=2,926) predicts relapse-free survival with 71% accuracy. Based on the aforementioned patients clinical and demographic risk factors, she is at very high risk for recurrence (>80% risk of relapse, 2-year recurrence-free survival of 23.8%):

With regards to adjuvant chemotherapy, the POUT trial has provided the first level-1 evidence.2 This trial was a phase III, parallel group, open-label, randomized controlled trial done at 71 National Health Service (NHS) hospitals in the United Kingdom. Eligible patients were ≥16 years, had received a radical nephroureterectomy for UTUC, were postoperatively staged with either muscle-invasive (pT2–pT4, pNany) or lymph node-positive (pTany, pN1–3) M0 disease with predominantly transitional cell carcinoma histology, and were fit to receive adjuvant chemotherapy within 90 days of surgery. Patients also had to have a glomerular filtration rate (GFR) of ≥30 mL/min. Prespecified stratification factors included platinum chemotherapy agent (cisplatin vs carboplatin), preoperative radiologically or pathologically assessed nodal involvement (N0 vs N1 vs N2 vs N3), status of surgical margins (positive vs negative), and treatment center. Patients were randomized 1:1 to receive either surveillance or adjuvant chemotherapy: four 21-day cycles of platinum-based chemotherapy (cisplatin 70 mg/m2) within 14 days of randomization; gemcitabine (1000 mg/m2) given on days 1 and 8 of each cycle. Patients with impaired renal function (GFR ≥30 mL/min but <50 mL/min) received carboplatin rather than cisplatin:
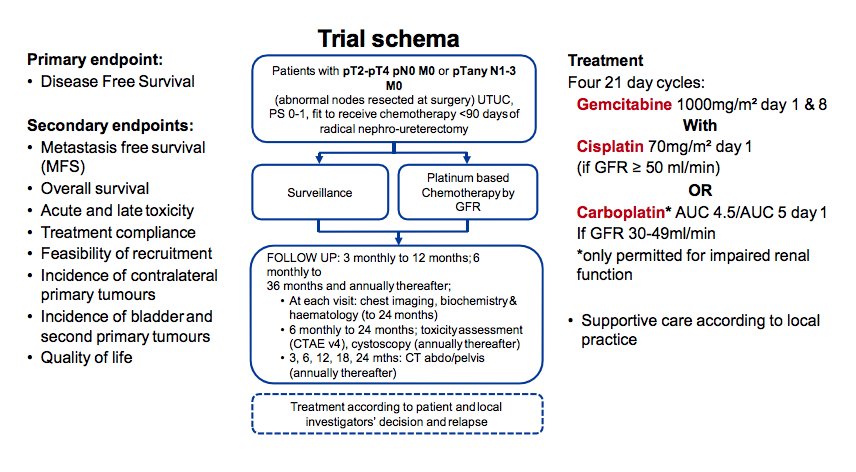
Patients were followed at 3, 6, 9, and 12 months, then every 6 months to 36 months from randomization, and annually thereafter. Radiographic assessment included CT of the thorax, abdomen, and pelvis at 3, 6, 9, 12, 18, 24, 30, and 36 months, then annually to 60 months. Cystoscopy was done every 6 months to 24 months, then annually up to 60 months after surgery. The primary endpoint of this trial was DFS defined as the time from randomization to either first recurrence in the tumour bed, first metastasis, or death from any cause. Secondary endpoints included metastasis-free survival, overall survival, treatment compliance, acute toxicity, late toxicity, and patient-reported quality of life.
There were 261 patients included in the trial between June 19, 2012, and November 8, 2017, at 57 of the 71 participating centers in the UK, including 129 patients randomized to surveillance and 132 to chemotherapy; 260 patients were included in the intention to treat analysis. Included patients were a median 68.5 years of age (IQR 62.0-74.1 years). With respect to tumor characteristics, 94% of patients had pT2-T3 disease and 91% were N0. The median follow-up was 30.3 months (IQR 18.0-47.5 months). There were 7 of 131 patients allocated to chemotherapy that did not start treatment and 75% of those that started chemotherapy received four cycles. There were 60 (47%) DFS events in the surveillance cohort and 35 (27%) in the chemotherapy cohort; as such, the unadjusted HR was 0.45 (95%CI 0.30-0.68) in favor of chemotherapy (log-rank p = 0.0001). The three-year disease-free survival rate was 46% for surveillance (95%CI 36-56) and 71% for chemotherapy (95%CI 61-78). Metastasis free survival also favored chemotherapy, with a HR of 0.48 (95%CI 0.31-0.74, log-rank p = 0.0007), and the three-year event-free rates were 53% (95% CI 42-63) for those on surveillance and 71% (95% CI 60-79) for those receiving chemotherapy:

Moderator: Surena F. Matin, MD, MD Anderson Cancer Center, Houston, TX
Panelists: Sima Porten, MD, MPH, University of California – San Francisco, San Francisco, CA & Vitaly Margulis, MD, UT Southwestern, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the AUA2021 May Kick-off Weekend May 21-23.
References:
- Krabbe LM, Eminaga O, Shariat SF, et al. Postoperative nomogram for relapse-free survival in patietns with high grade upper tract urothelial carcinoma. J Urol. 2017 Mar;197(3 Pt 1):580-589.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020 Apr 18;395(10232):1268-1277.


