Following an introduction to genomic testing from Dr. Wagner and his own presentation on pre-diagnostic markers, Dr. Cooperberg discussed the role of germline testing in patients with prostate cancer. As Dr. Wagner had done in his initial introduction, Dr. Cooperberg began with an introduction to genomic testing, emphasizing the difference between somatic mutations (affecting any cell of the body apart from germ cells, but most typically tumor cells in this context) and germline mutations (affecting all cells in the body, including germ cells, which may be transferred to offspring). As expected from the name, germline tests examine mutations that are inherited and may be detected in any cell. Thus, these tests typically are performed on blood, saliva, buccal swab, or other non-tumor sources. In contrast, genomic tests look for somatic mutations within the tumor. Notably, some tests which are grouped within this category do not detect mutations, but rather examine other changes including DNA methylation and protein expression.
The role of germline testing is rapidly growing and the results becoming increasingly actionable, particularly in men with advanced disease. A number of recent efforts have sought to define the role of genetic testing in prostate cancer, including the Philadelphia consensus conference. While there has been a heavy focus on BRCA2 and ATM, there are many other germline mutations of interest.
Further, over time, indications for germline testing in prostate cancer have become increasingly broad over time, encompassing the whole spectrum of prostate cancer, depending on disease characteristics and family history. Dr. Cooperberg emphasized that the family history necessary to mandate germline testing involves not just an affected family member but one of the following:
- A family history of germline mutations including BRCA, Lynch syndrome, and others
- Ashkenazi Jewish ancestry
- A family history of prostate cancer involving a first-degree relative or multiple relatives with early diagnosis or aggressive disease including lethal disease
- Other suggestion of familial cancer syndromes including three or more relevant cancers (colon, urothelial, breast, pancreas, ovarian, or prostate cancer) on the same side of the family.
He further emphasized that “harder we look, the more we find” in terms of germline mutations in prostate cancer patients. While we have known for years that prostate cancer is among the most heritable cancers, it has taken a long time to characterize the underlying genetic causes as there are not single driving events or mutations which account for a large proportion of disease. However, work from Pritchard and colleagues and from The Cancer Genome Atlas have shown that nearly 12% of men with mCRPC and 6% of men with localized high-risk disease have recognized underlying germline mutations associated with prostate cancer. In addition to these recognized mutations, there are clearly many causative genetic changes that are not identified, in part due to polygenic inheritance of prostate cancer and the potential for familial meta-genetic factors which are not captured in these types of analyses.
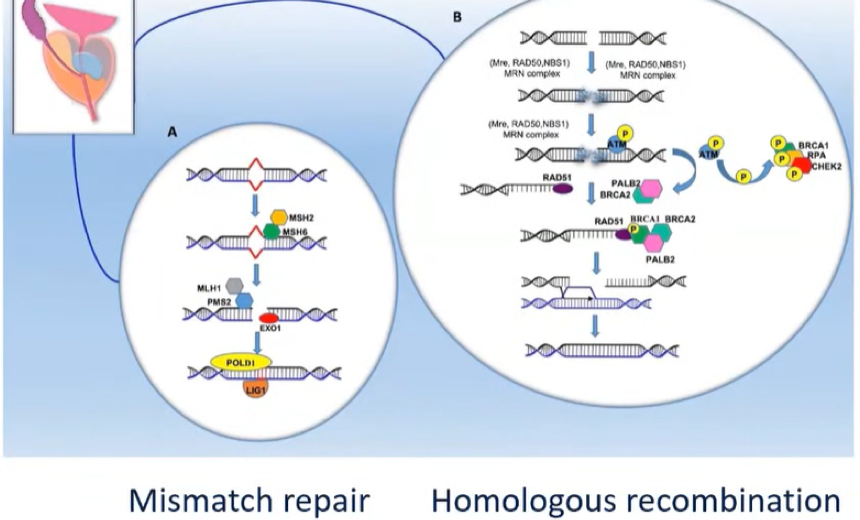
As alluded to above, the importance of germline testing is growing as identification of these mutations is becoming increasingly actionable: mutations in homologous recombination repair may confer susceptibility to PARP inhibitors and platinum-based chemotherapy while mutations in DNA mismatch repair genes may confer susceptibility to immune checkpoint inhibitors.

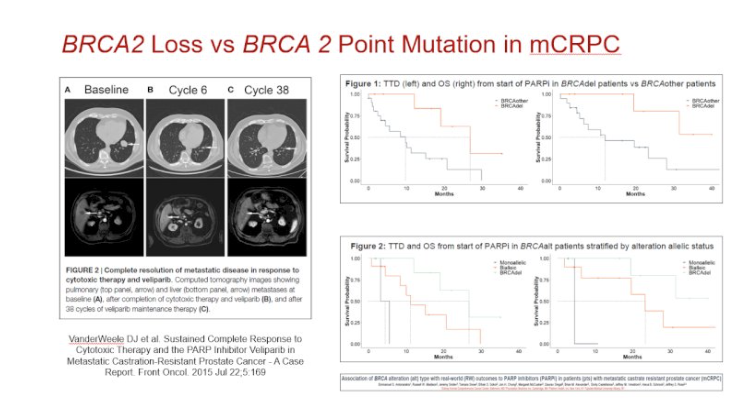
In patients with identified BRCA mutations, Dr. Cooperberg then considered the question of whether they should receive different treatment recommendations than commonly provided in guidelines. Certainly, there is evidence of an increased risk of metastasis and prostate-cancer-specific mortality in patients with localized prostate cancer who have BRCA mutations. However, the question is whether this information can or should influence treatment decisions is unclear. Recent data from Johns Hopkins suggests that patients with BRCA mutations are more likely to have upgrading or progression to definitive treatment while on active surveillance.
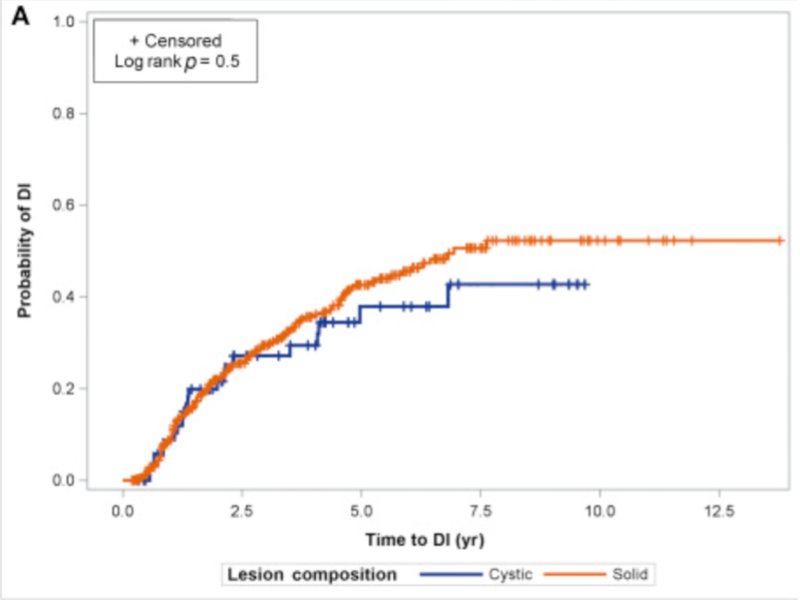
However, at 6-years, nearly half of all men with BRCA mutations will be free of progression. Thus, Dr. Cooperberg suggested that we can still offer surveillance to these men, as long as surveillance regimes are particularly stringent to allow for timely identification of progression and timely treatment.
He then moved to discuss treatment implications of germline mutations, first focussing on mutations in the homologous recombination repair pathway. In the TRITON2 study, approximately 40% of patients with BRCA1 and BRCA2 responded to the PARP inhibitor rucaparib. However, this demonstrates that, even among these patients selected based on germline mutations, there is not an “all or nothing” benefit, reflecting the lack of specificity of treatment and of markers and emphasizing the need for better companion diagnostics. In addition to rucaparib which was assessed in TRITON2, olaparib was examined in the randomized PROfound trial demonstrating benefit, particularly in patients with mutations in BRCA1, BRCA2, and ATM. Moving forward, PARP inhibitors are actively being studied in the hormone-sensitive disease space in the recently launched phase III AMPLITUDE trial of abiraterone plus niraparib versus abiraterone alone for patients with mutations in homologous recombination repair genes.
In addition to the treatment implications of mutations in homologous recombination repair genes, mutations in DNA mismatch repair and microsatellite instability confer sensitivity to immune checkpoint inhibition and indicate eligibility for the PD-1 inhibitor pembrolizumab.
In addition to the biological rationale treatments, there is ongoing work to identify and test other biologically driven treatment approaches to allow for rationale treatment based on tumor biology. For example, among patients with deletions or mutations in PTEN or with activation of the PI3-kinase or AKT pathways, treatment with the oral AKT inhibitor ipatasertib was tested in the phase III IPATential150 trial and may lead to FDA approval given the positive results of this trial.
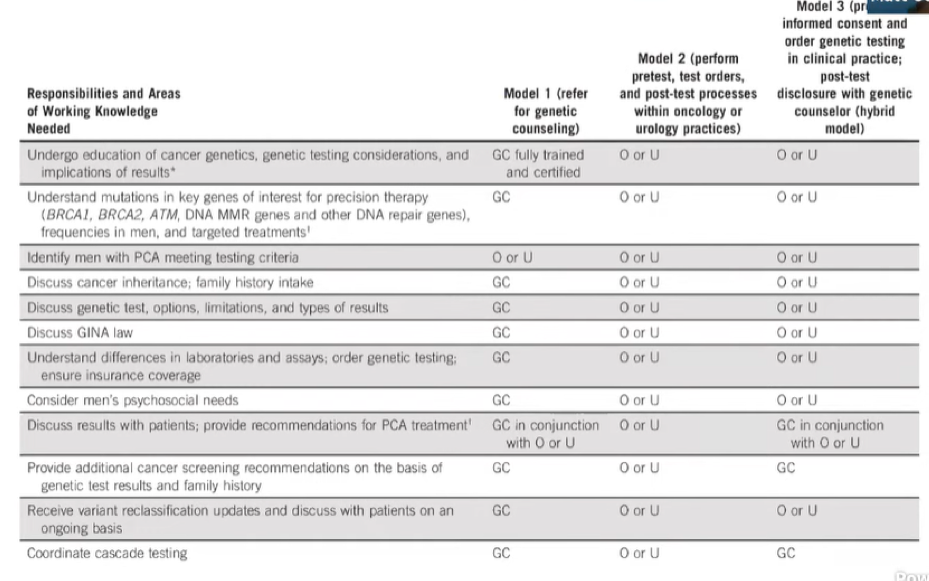
Stepping back for a minute, Dr. Cooperberg considered the question of whether we are testing enough patients. Citing a recent analysis, he suggested that current guidelines may be too restrictive: among 3607 men with prostate cancer referred for genetic testing, 620 (17%) had identified germline variants. A relatively large proportion of these men (n=229, 37%) with identified germline mutations would not have been approved for genetic testing on the basis of existing NCCN guideline recommendations. However, more widespread use of genetic testing opens the question of how to undertake this: options include a model with early referral to genetic counseling, a model in which the clinician takes ownership for much of the process including ordering the test and discussing the results, and a hybrid model.
For clinicians considering ordering genetic testing, it is important to understand what each test examines: while usually, these assays examine a relatively limited subset of cancer-related genes, relevant genes for prostate cancer are typically included.
In conclusion, Dr. Cooperberg emphasized the importance of good family history which, along with pathology, can guide the identification of appropriate patients for germline testing. Further, he suggested that clinicians should establish an algorithm for testing and counseling, including a relationship with genetic counselors.
Presented by: Matthew Cooperberg, MD, MPH, FACS, Professor of Urology; Epidemiology & Biostatistics, Helen Diller Family Chair in Urology, The University of California, San Francisco, UCSF
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter during the AUA2021 May Kick-off Weekend May 21-23


