(UroToday.com) The 2024 American Urological Association (AUA) Annual Meeting held in San Antonio, TX between May 3rd and 6th, 2024 was host to the International Prostate Forum. Dr. Angela Jia presented updates on metastasis-directed therapy (MDT).
The indications for radiotherapy in prostate cancer have significantly increased over the last decade. Summarized below are the current indications for radiotherapy in prostate cancer: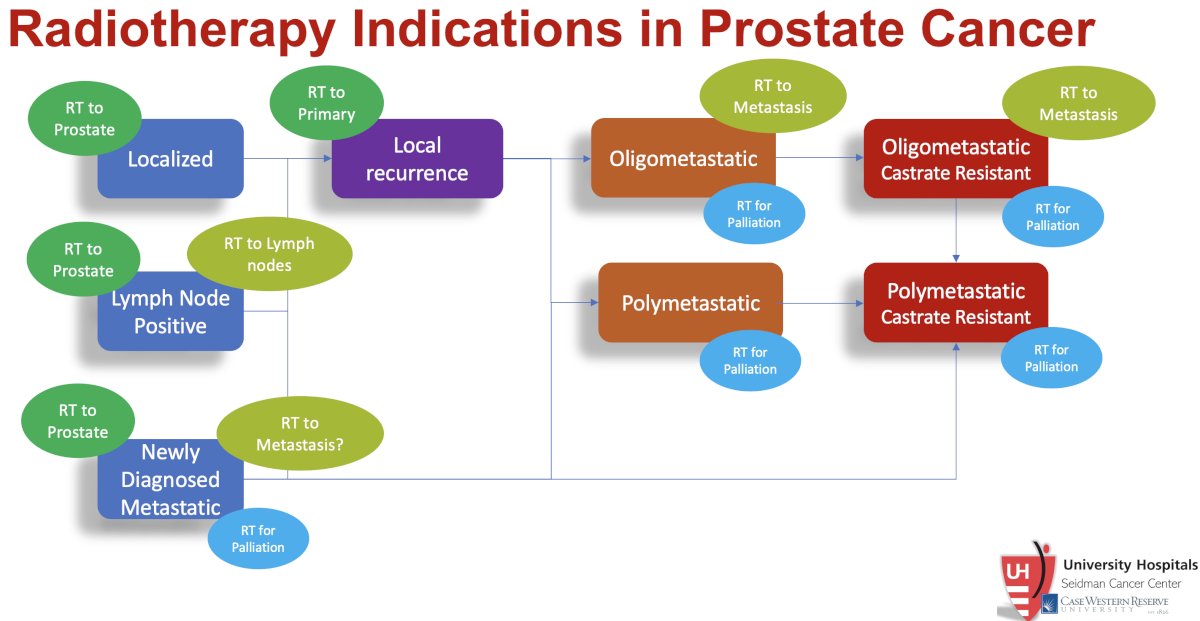
What are the specific indications for stereotactic ablative radiotherapy (SABR)? Metastasis-directed therapy with SABR to the sites of metastatic disease is being evaluated in patients with de novo (i.e., synchronous) and recurrent (i.e., metachronous) hormone-sensitive and castrate-resistant disease.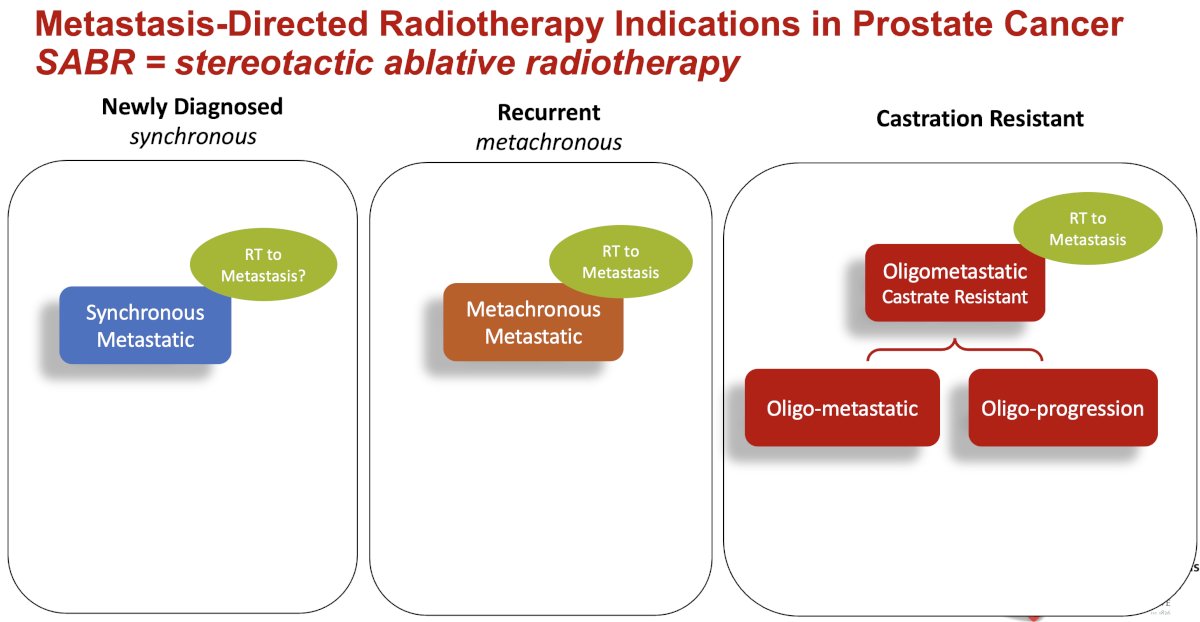
MDT is not a concept unique to prostate cancer. As summarized in the table below, there have been numerous studies evaluating SABR to metastatic lesions in other disease sites such as non-small cell lung, colorectal, esophageal, and breast cancers, among others. Trials of MDT in prostate cancer have included STOMP, ORIOLE, EXTEND, ARTO, and SABR-COMET (included patients with numerous malignancies, including prostate). SABR has consistently shown benefits across these cancer types. The objective of MDT is analogous to that of chemotherapy – cytoreduction; however, Dr. Jia argued that SABRT offers a great progression-free survival benefit with significantly fewer side effects.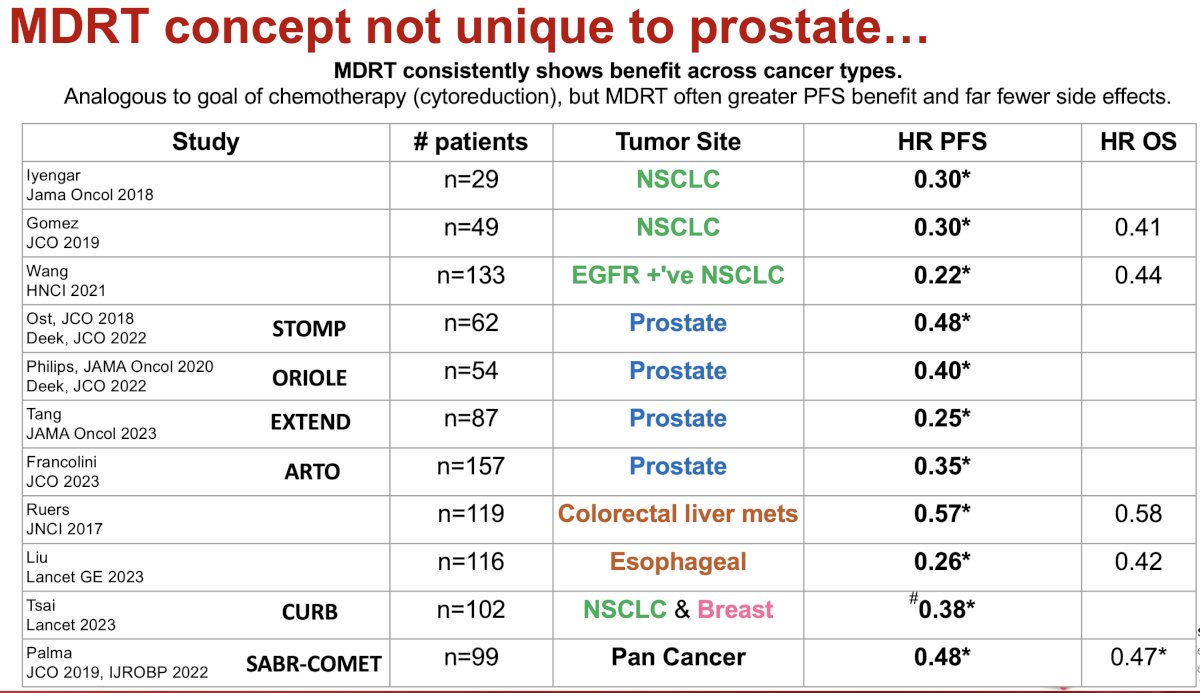
The benefits of total consolidation, like most forms of systemic therapy, are aimed at reducing or eliminating metastasis to reduce or prevent metastasis seeding. Both the primary tumor and metastasis sites are sources of potentially new metastatic disease. Thus, targeting, the metastatic sites, along with the primary, may theoretically help reduce tumor ‘shedding’ and disease progression.
Concurrent with the emergence of SABR for oligometastatic prostate cancer, we have witnessed an evolution in the definition of oligometastatic prostate cancer. Historically, oligometastatic disease referred to 1–3, novel, non-visceral metastatic sites. This definition has been expanded in several trials to include up to 10 metastatic sites total, potentially including visceral sites of disease and new/progressive sites of disease. There has been a shift away from defining the ‘upper limit’ of oligometastatic disease by the number of metastatic lesions. Instead, the majority of participants from the ESTRO-ASTRO consensus conference agreed that the ‘upper limit’ of oligometastatic disease should be defined by the ability to safely deliver curative intent metastasis-directed radiotherapy.
The objective of MDT has similarly evolved over the past decade. Classically, MDT has been reserved for patients that may potentially still be curable with such an approach. Currently, MDT is still administered with a potentially curative intent; however, additional indications potentially include providing highly effective cytoreduction to improve progression-free survival, eliminating resistant clones, and ultimately improving quality and quantity of life.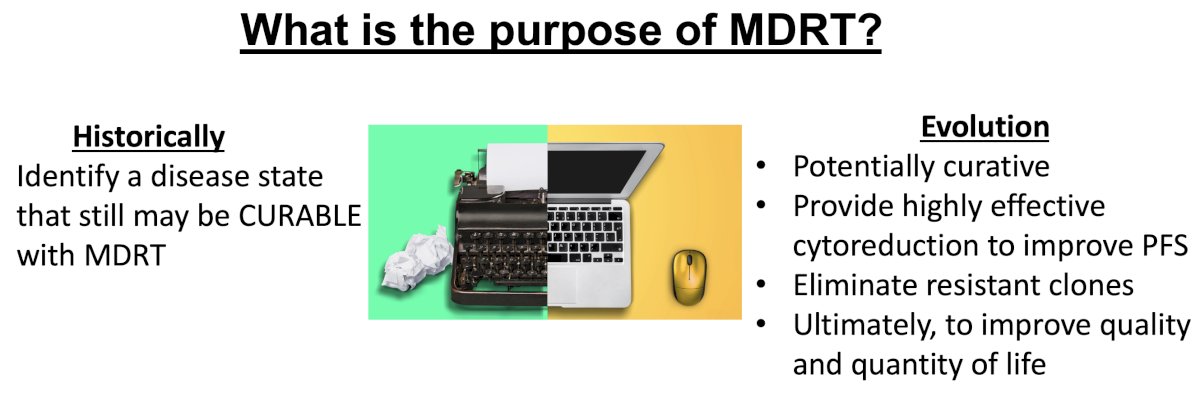
Summarized below are the results of five major trials of SABR in oligometastatic prostate cancer patients.
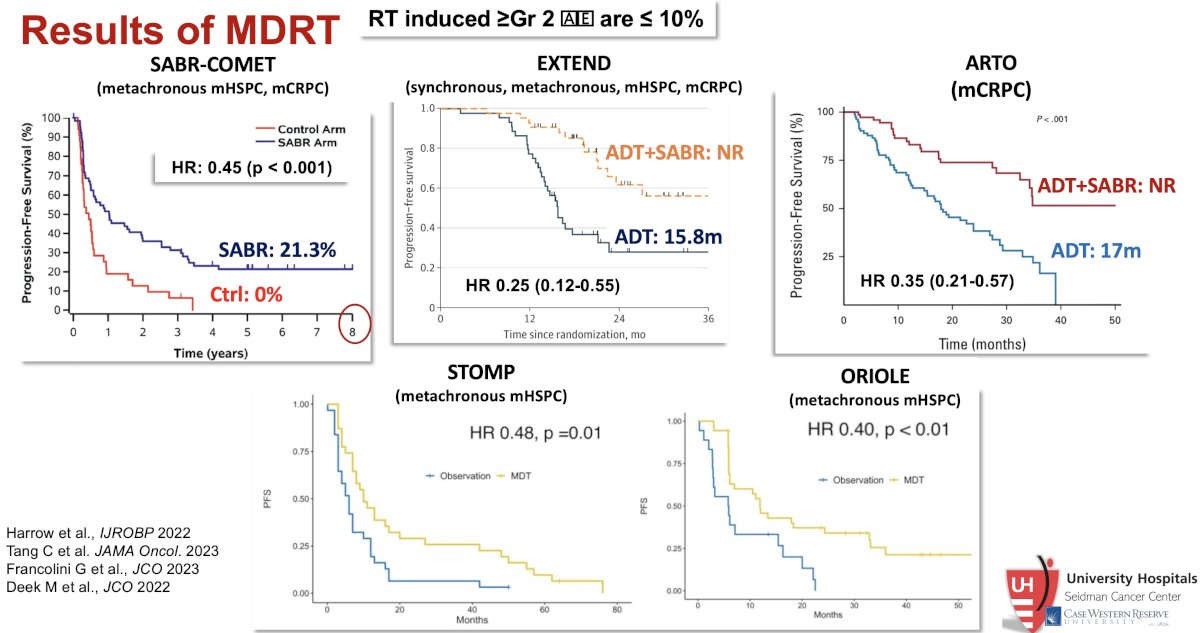
SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (182%) had prostate cancer. After stratifying by the number of metastases (1–3 vs 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SABR. In an updated analysis published in 2020 (median follow-up 51 months), the five-year overall survival rate was 17.7% in the control arm and 42.3% in the SABR arm (stratified log-rank p=0.006). The corresponding median overall survivals were 28 months and 50 months, respectively. There were no new grade 2-5 adverse events and no differences in quality of life between the arms.1,2
The STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for patients with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence with testosterone >50 ng/ml (i.e. metachronous, oligometastatic mHSPC). Between 2012 and 2015, 62 patients were randomized 1:1 and MDT was either SABR or metastasectomy. The primary endpoint was time to initiation of ADT (called ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease. Time to castration resistance was a secondary endpoint (called CRPC-free survival). The updated five-year results were presented at GU ASCO 2020. With a median follow-up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38-0.84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases. The secondary endpoint of CRPC-free survival at 5 years was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI: 0.35-1.09).3
The ORIOLE trial was a randomized phase II trial of 54 men with metachronous, oligometastatic mHSPC (up to three sites). Metastatic sites were diagnosed via conventional imaging. Between 2016 and 2018, patients were randomized in a 2:1 fashion to receive SABR or observation. The primary outcome was progression at 6 months, defined as serum PSA increase, progression detected by conventional imaging, symptomatic progression, ADT initiation for any reason, or death. Progression at six months occurred in 7 of 36 patients (19%) receiving SABR and 11 of 18 patients (61%) undergoing observation (p=0.005). Treatment with SABR improved median PFS (not reached vs 5.8 months; hazard ratio, 0.30; 95% CI, 0.11-0.81; p=0.002). No toxic effects of grade 3 or greater were observed.4
The EXTEND trial was a single-center, phase II randomized controlled trial of 87 oligorecurrent men, mostly with mHSPC (>90%), who were randomized 1:1 to intermittent hormone therapy +/- MDT (definitive radiation therapy to all sites of disease). All patients had ≤5 metastases, as defined by conventional imaging (75%) or fluciclovine PET/CT (25%). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression. At a median follow-up of 22 months, progression-free survival was improved in the combined therapy arm (HR: 0.25, 95% CI: 0.12 – 0.55, p<0.001). Significantly, ‘eugonadal’ progression-free survival was also improved with this combination approach (HR: 0.32, p=0.03).5
Shifting to the mCRPC setting, ARTO was a multicenter, phase II trial of SABR addition to abiraterone plus prednisone in patients with oligometastatic CRPC. This trial included 157 patients, enrolled between January 2019 and September 2022. A biochemical response, defined as a 6-month PSA decrease ≥50% from baseline, was achieved in 92% of SABR-treated patients, versus 68.3% of patients receiving systemic therapy alone (OR: 5.34, 95% CI: 2.1 – 13.9, p=0.001). SABR yielded a significant PFS improvement, with a hazard ratio for progression of 0.35 (95% CI: 0.21 to 0.57; p<0.001) in the experimental versus control arm.6
Significantly, SABR appears to have a favorable safety profile. A recent systematic review and meta-analysis of SABR in patients with oligometastatic prostate cancer demonstrated that serious adverse events are very unlikely.7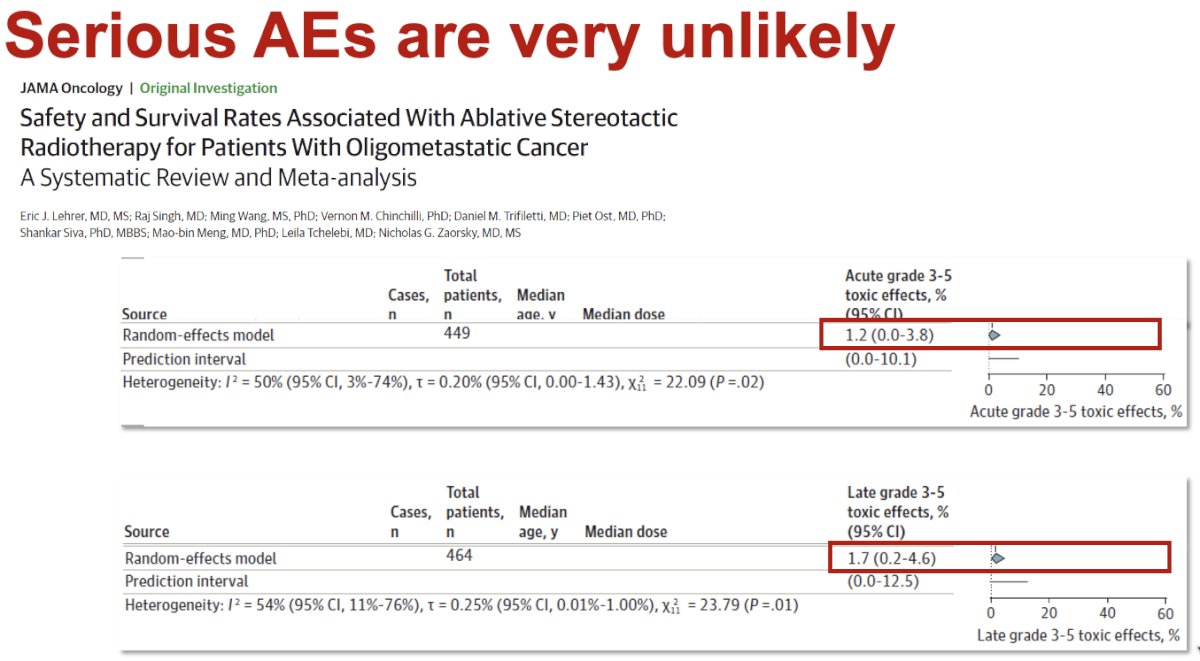
Should MDT-treated patients undergo treatment intensification with the addition of ADT/ARPI? This depends on the goal of care:
- Patient desires to avoid hormonal therapy: Likely MDT alone (and repeat as needed)
- Maximize treatment-free interval: Likely add ADT to MDT (and repeat as needed)
- Eliminate resistant clones: Add ADT/ARPI to MDT
Following MDT with SABR, potential outcomes are as follows: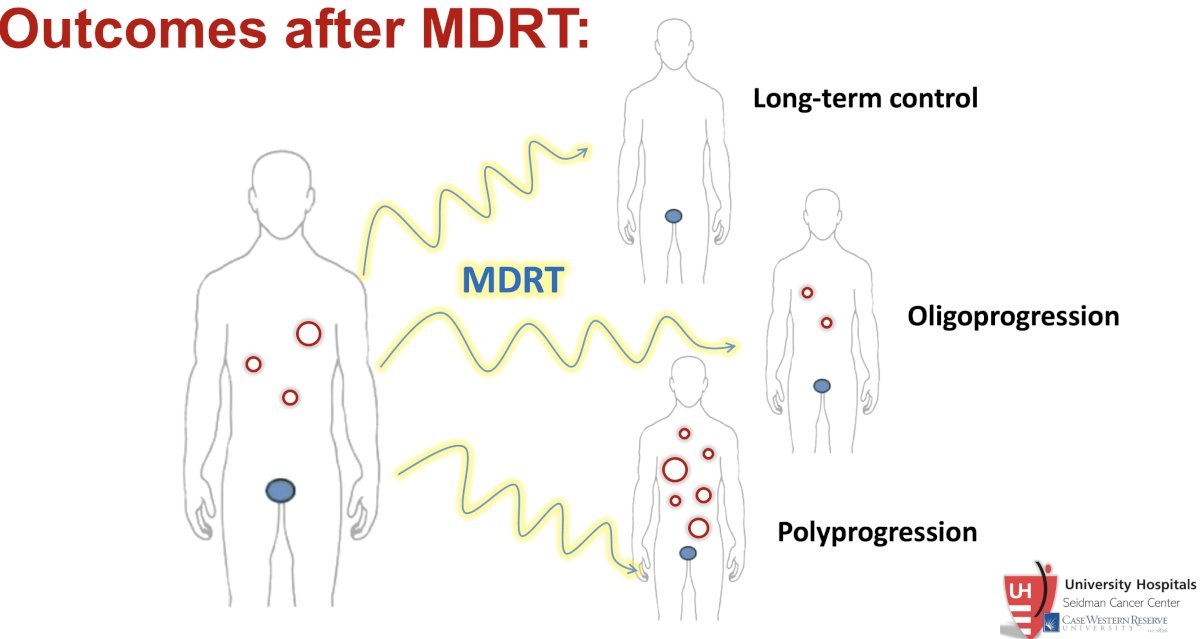
The addition of ADT to MDT appears to improve long-term control but does not reduce the rates of polyprogressive disease. In 2020, Deek et al. demonstrated that the addition of ADT to SABR improves long-term control of primary secondary disease sites from 28% to 41%. However, the proportions of patients experiencing polyprogression remained relatively similar (27.6% versus 23%).8
Ongoing randomized controlled trials of MDT with SABR in oligometastatic prostate cancer are summarized below: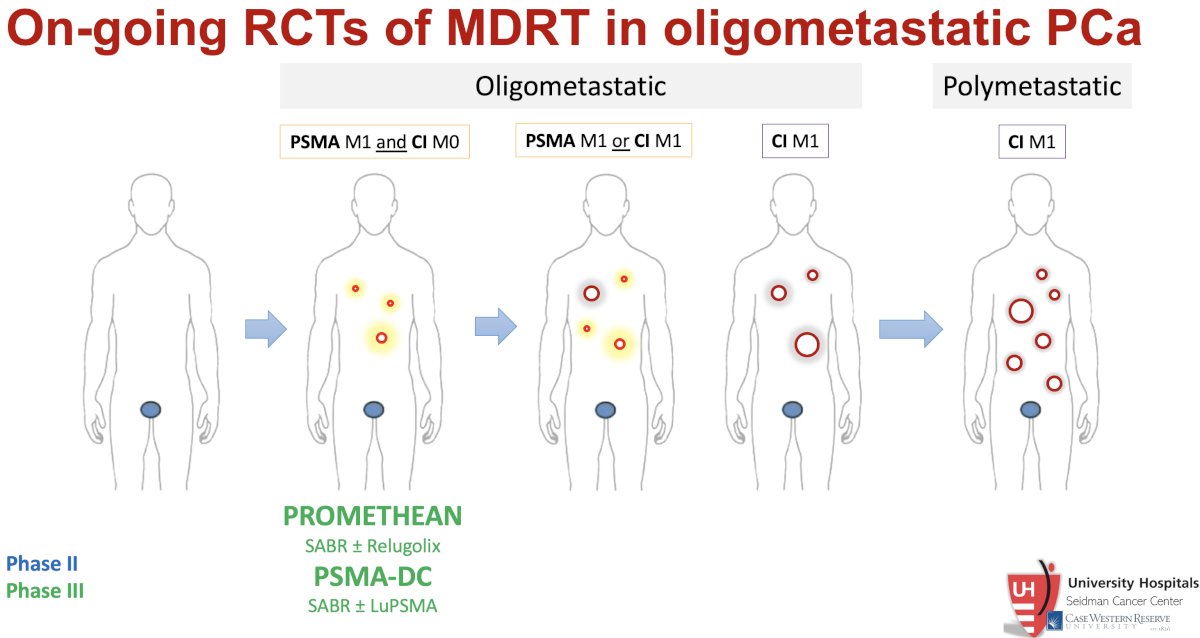
Summarized below are those trials specifically evaluating SABR in patients with de novo metastatic disease: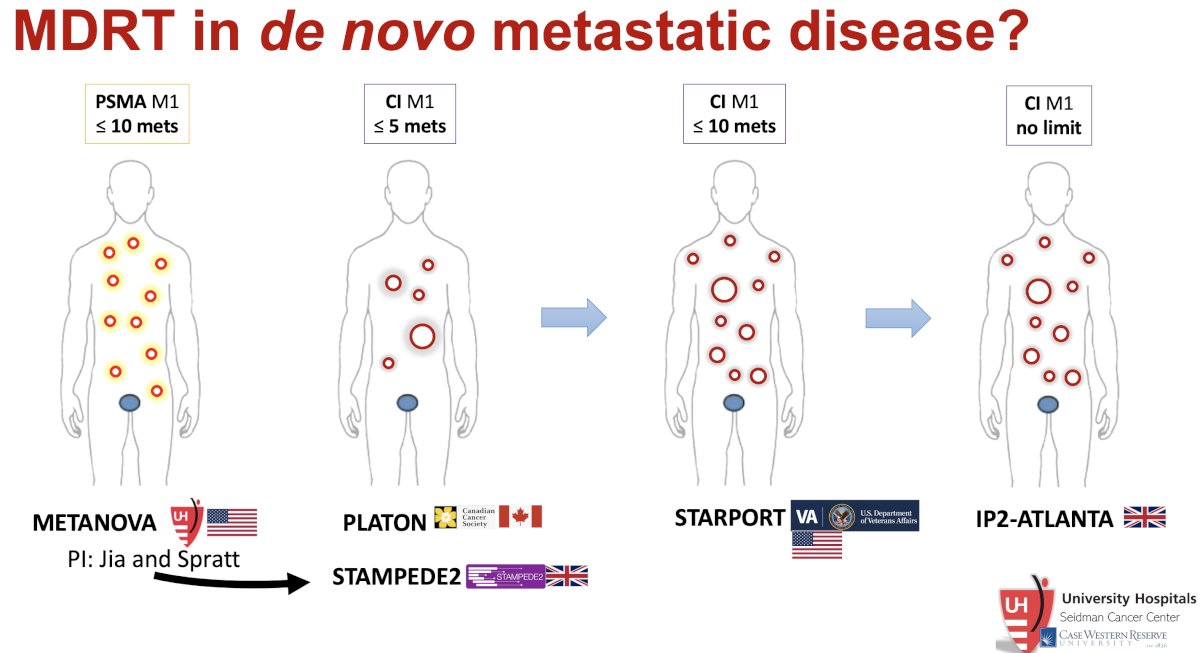
Can we personalize the use of SABR for prostate cancer patients? In the ORIOLE trial, treating all PSMA-PET avid lesions was associated with significantly improved distant metastasis-free survival, compared to leaving some lesions untreated (HR: 0.19, p<0.001).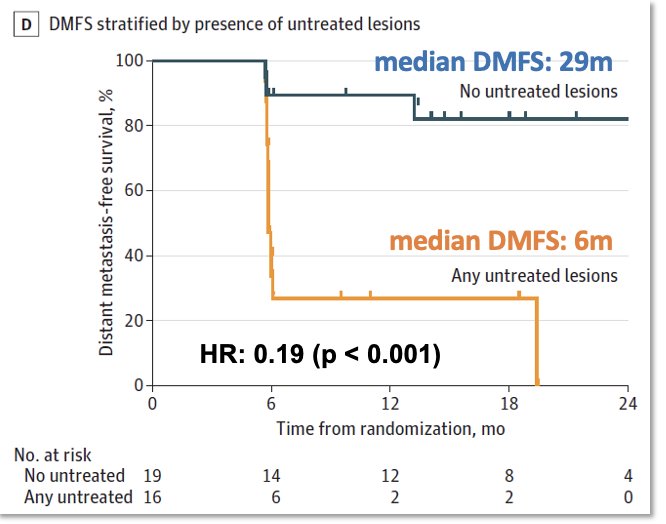
In a pooled analysis from ORIOLE and STOMP, oligorecurrent prostate cancer patients with high-risk mutations (ATM, BRCA1/2, RB1, or TP53) derived a greater benefit from SABR.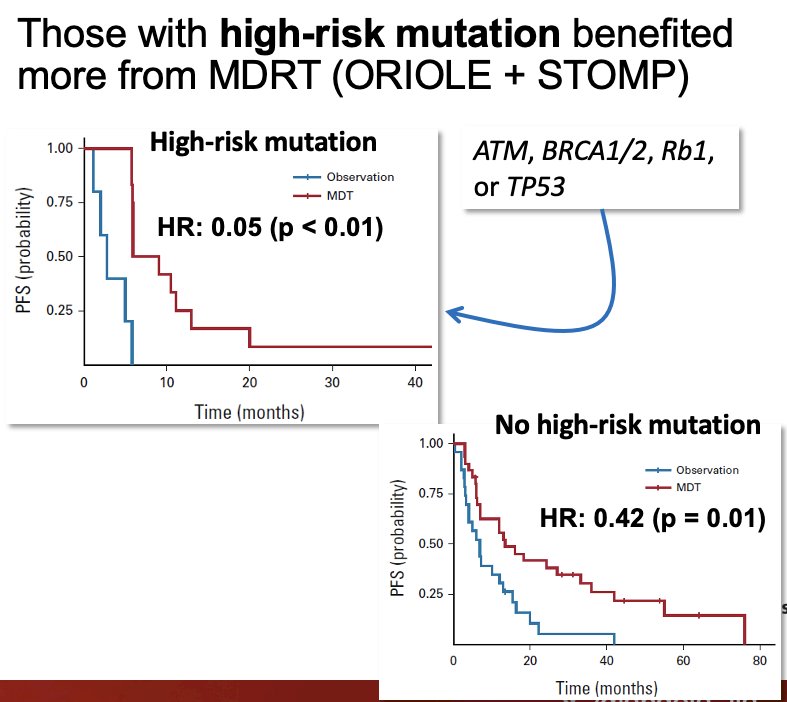
As such, use of PSMA-PET and genomic profiling may help predict improved response to SABR. There are currently no validated predictive biomarkers in this space, which is not dissimilar to guiding use of chemotherapy, ARPIs, etc. Ongoing large, randomized trials will enable training/validation of biomarkers in this space.
Dr. Jia acknowledged that many misconceptions about SABR remain, particularly with regard to prognosis and benefit. Many advocate restricting SABR to only the most favorable patients. Similar to localized disease, these are patients that would have relatively favorable outcomes to observation. The largest benefits of SABR are found in non-small cell lung cancer and high-risk prostate cancer. She argued that we need to expand our view of what MDT is and its purpose:
- It can be to render some patients disease-free
- Can also be a highly effective form of cytotoxic therapy delivered precisely to gross disease with minimal toxicity
- Similar to other cytotoxic therapies (177Lu-PSMA, chemotherapy), which are also not curative in advanced disease
Dr. Jia concluded with the following take-home messages:
- Treatment of metastatic sites with radiotherapy improves progression-free survival in oligo recurrent disease
- 15-20% of patients treated with MDT have long-term disease-free intervals
- Limited data in de novo mHSPC
- Treatment of all sites of metastatic disease (5-10) is feasible, well-tolerated, and prolongs ADT-free survival.
- Optimal duration and sequencing of ADT with local therapy is unknown.
- Numerous ongoing trials: NRG GU011, DART (NCT04641078), RADIOSA (NCT03940235)
- Oligorecurrent disease is a heterogeneous population, currently defined by imaging sensitivity. Molecular stratification is needed to determine who will benefit from MDT ± ADT.
Presented by: Angela Jia, MD, PhD, Assistant Professor, Department of Radiation Oncology, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, OH
Written by: Rashid Sayyid, MD, MSc - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, May 3rd - 6th, 2024
References:- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185): 2051-8.
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38(25) :2830-8.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5) :446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate CancerThe ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5): 650-659.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6): 825-834.
- Francolini G, Allegra AG, Detti B, et al. Stereotactic Body Radiation Therapy and Abiraterone Acetate for Patients Affected by Oligometastatic Castrate-Resistant Prostate Cancer: A Randomized Phase II Trial (ARTO). J Clin Oncol. 2023;41(36) :5561-8.
- Lehrer EJ, Singh R, Wang M, et al. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021;7(1): 92-106.
- Deek MP, Taparra K, Dao D, et al. Patterns of Recurrence and Modes of Progression After Metastasis-Directed Therapy in Oligometastatic Castration-Sensitive Prostate Cancer. Int J Radiat Oncol Biol Phys. 2021;109(2): 387-95.


