(UroToday.com) The 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3 and May 6, 2024, was host to the Society of Urologic Oncology (SUO) session Dr. Robert Svatek gave a brilliant talk on immunology in non-muscle invasive bladder cancer (NMIBC) and the role of immunology in neoadjuvant therapy for muscle-invasive bladder cancer (MIBC).
Dr. Svatek began his presentation by discussing how the field of bladder cancer immunology and immunotherapy has exploded in the last couple of decades. He shared a slide featuring the FDA-approved agents in bladder cancer, which are steadily growing. Currently, there are over 17 FDA-approved agents in bladder cancer.
He discussed how chemotherapy could be similar to indirect immunotherapy and he highlighted 4 mechanisms of action of chemotherapy that directly affect the immune system:
- Immunogenic Cell Death: Triggered by tumor antigens and "danger signals"
- Mitigating Immunosuppressive cells: Chemotherapy can kill regulatory T cells (Tregs)
- Activating T cells: Gemcitabine enhances dendritic cell cross-presentation of tumor antigen to CD8+ T cells
- Sensitization of cells to T cell killing: Cisplatin - makes cells more permeable to granzyme
A comparison was made between innate immunity and adaptive immunity. Innate immunity typically involves a rapid, nonspecific response mediated by macrophages, natural killer cells (NK), and dendritic cells (DC), while adaptive immunity initiates a slower but highly specific response to an antigen. Adaptive immunity is usually mediated by T cells (helper and cytotoxic) and B cells. Notably, both innate and adaptive immunity involve T cells and natural killer cells in their responses.
The adaptive immune response is mediated by T cells and antigen-presenting cells (APCs), these cells interact through a process called antigen presentation. APCs, such as dendritic cells, macrophages, and B cells, capture antigens from pathogens or other foreign substances and process them into smaller peptides. These peptides are then displayed on the surface of the APCs bound to major histocompatibility complex (MHC) molecules. When T cells encounter APCs presenting antigen-MHC complexes that match their specific T cell receptor (TCR), they become activated. This activation process involves multiple interactions and signaling events. Dr Svatek mentioned that co-stimulatory molecules on the surface of the APC, such as CD80/86, bind to co-stimulatory receptors, such as CD28 and CTLA-4 on the surface of the T cell. This interaction provides the second signal necessary for full T cell activation and proliferation. Once activated, T cells differentiate into effector T cells, such as cytotoxic T cells or helper T cells.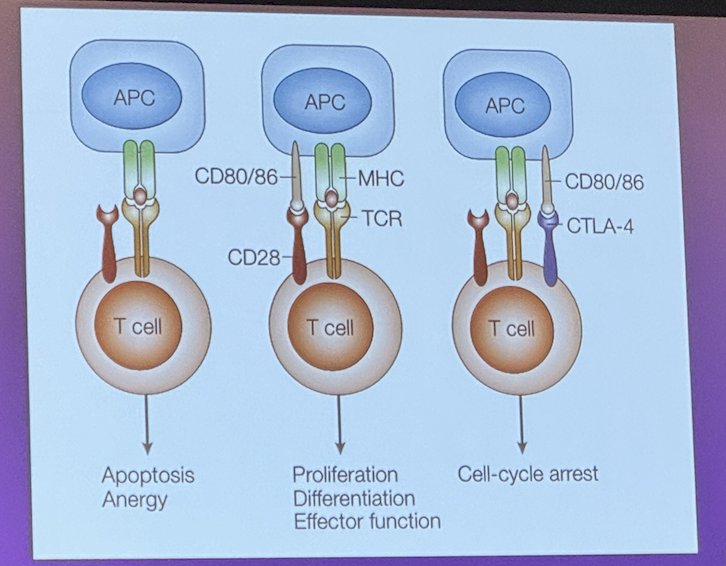
The PD-1 pathway, also known as the programmed cell death protein 1 pathway, is a key regulatory mechanism in the immune system that plays a critical role in controlling and inhibiting T-cell activation and maintaining immune tolerance through 4 mechanisms:
- Reduced TCR Signaling
- Reduced Cytokine Production
- Reduced Target Cell Lysis
- Metabolic Reprogramming
Furthermore, PD-1 is highly expressed in tumor-infiltrating T cells (TIL) and these are functionally exhausted. PD-1 or PD-L1 Blockade can revive exhausted TILs, enhancing their function to recognize and attack tumor cells (IFN-Y)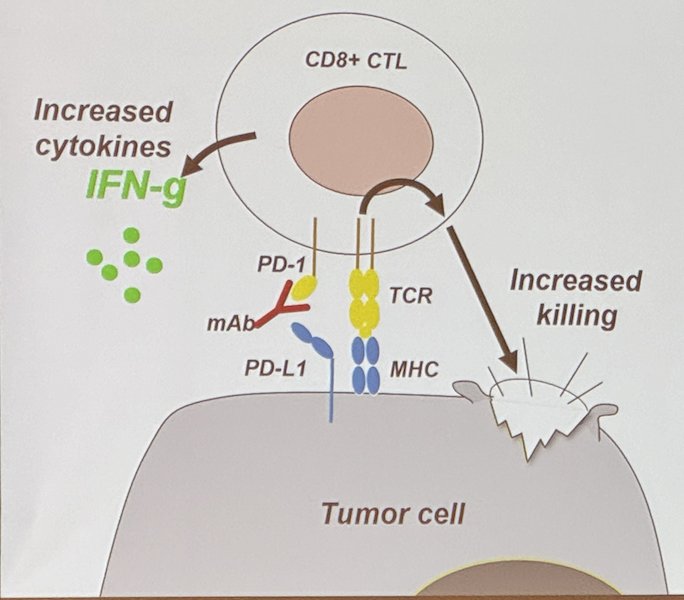
Additionally, there is another key player in immunology for bladder cancer. This is the NK cell. Bacillus Calmette Guerin (BCG), one of the most commonly used intravesical therapies for bladder cancer, acts particularly through the activation of NK cells. BCG is taken up by antigen-presenting cells (APCs), such as macrophages and dendritic cells, which then present BCG-derived antigens to T cells. This activates T cells, leading to the production of cytokines such as interferon-gamma (IFN-γ). IFN-γ, in turn, activates NK cells, which play a crucial role in the innate immune response against tumor cells by inducing apoptosis and releasing cytotoxic granules. These cytotoxic granules contain perforin and granzymes towards tumor cells. Perforin creates pores in the tumor cell membrane, allowing granzymes to enter and induce apoptosis, a process of programmed cell death. Granzymes activate caspases within the target cell, leading to DNA fragmentation and cell death.
Additionally, NK cells express death receptors such as Fas ligand (FasL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which engage with corresponding receptors on tumor cells, initiating apoptotic signaling pathways.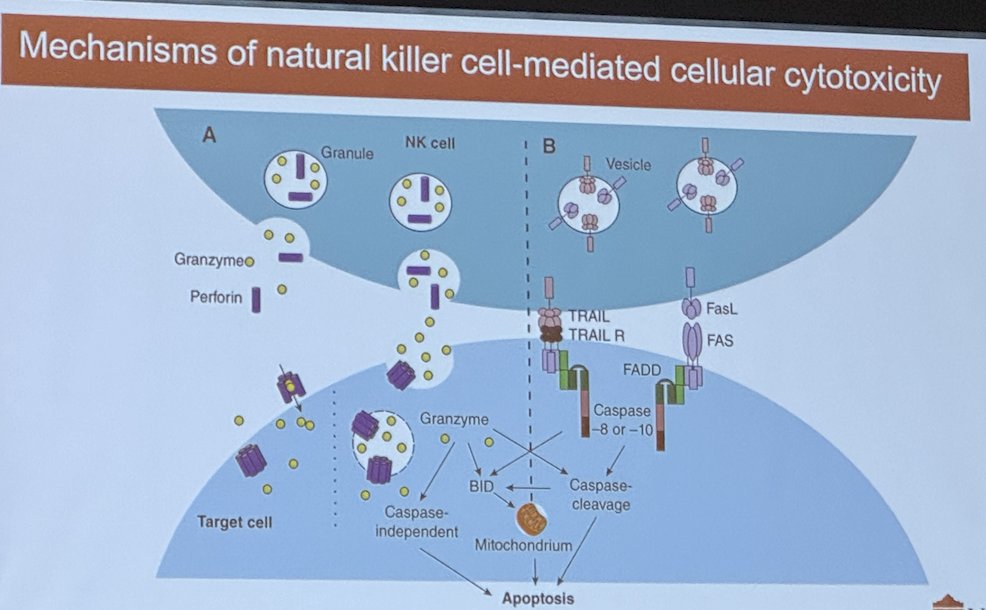
In a study assessing the prevalence of intratumoral via flow cytometric analysis of bladder tissue from 28 patients with non-invasive and invasive bladder cancer, the investigators found that NK cells predominated among bladder intratumoral lymphocytes. Intratumoral CD56bright NK cells showed increased cytokine production and cytotoxicity compared to their CD56dim counterparts and were associated with improved cancer-specific (CSS) and overall survival (OS) independent of pathologic tumor stage.1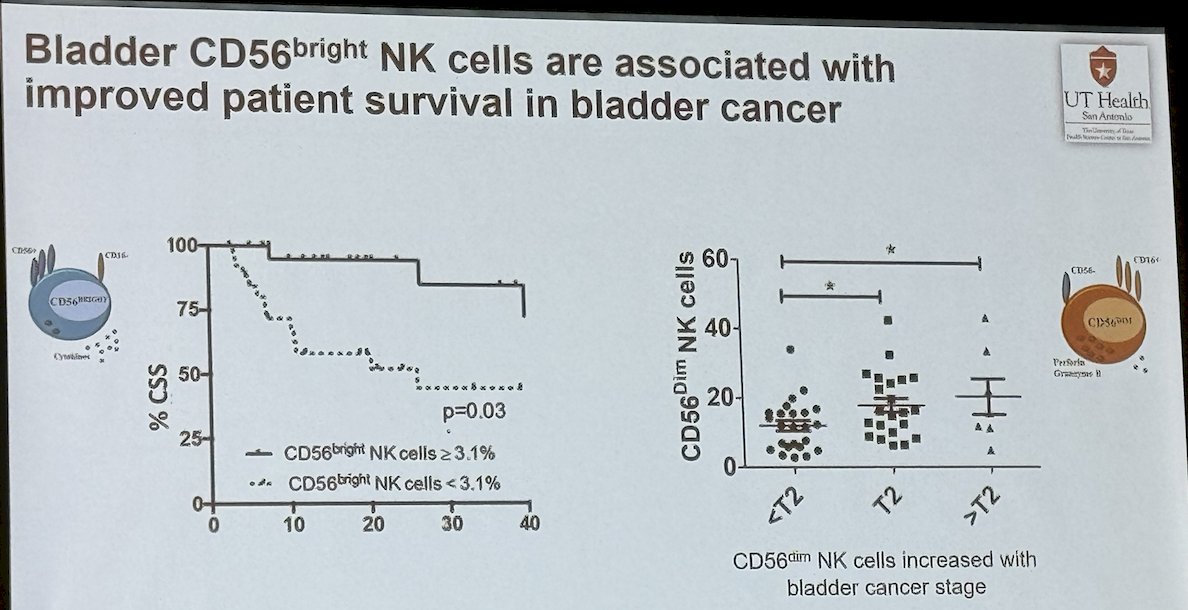
Moreover, intratumoral CD56bright NK cells produced more IFN-γ and were more cytotoxic (% cell death) than intratumoral CD56dim NK cell.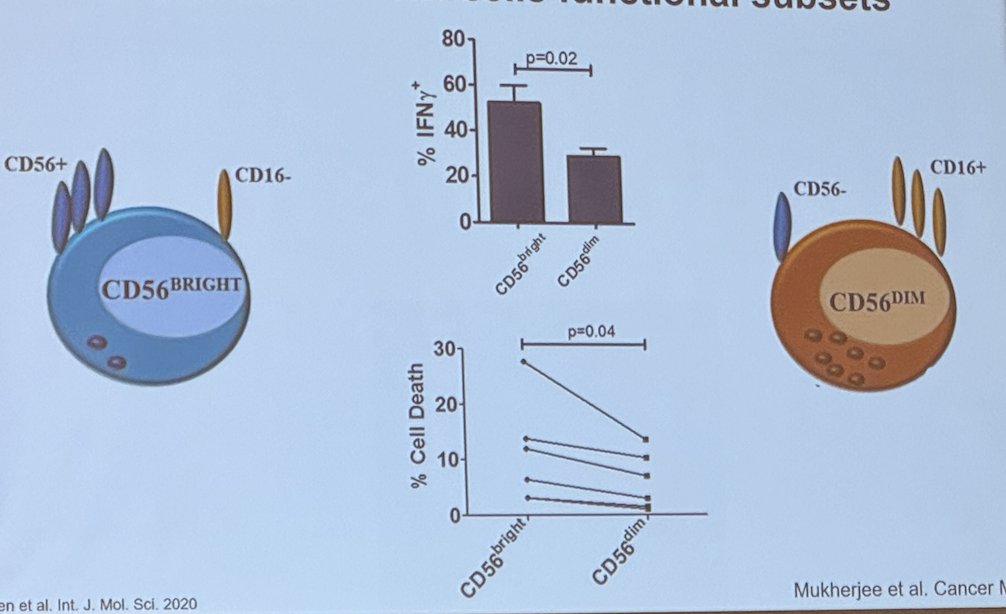
Dr. Svatek concluded his presentation with the following key points:
- Successful cancer therapy is equal to immunotherapy.
- We have to understand the principles of T cell cytotoxicity, including memory, which determines the durability of response, and specificity, which minimizes the side effects of immunotherapy.
- The T cell costimulatory receptor CD28 is a primary target of PD-1/PD-L1 antibodies. NK cell cytotoxicity, when highly regulated, prevents autoimmunity against tumoral cells, and it can also be "trained" through epigenetic or metabolic programming.
- The presence or absence of immune cells does not tell you about their function.
Presented by: Robert Svatek, MD, MSCI, Urologic Oncologist, UT Health San Antonio, San Antonio, TX
Written by: Julian Chavarriaga, MD - Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Urological Association (AUA) annual meeting held in San Antonio, TX between May 3rd and May 6th, 2024
Reference:
- Mukherjee N, Ji N, Hurez V, Curiel TJ, Montgomery MO, Braun AJ, Nicolas M, Aguilera M, Kaushik D, Liu Q, Ruan J, Kendrick KA, Svatek RS. Intratumoral CD56bright natural killer cells are associated with improved survival in bladder cancer. Oncotarget. 2018 Nov 23;9(92):36492-36502.


