(UroToday.com) John Wei presents this year’s update on the AUA Guidelines, focusing on the Early Detection of Prostate Cancer. He concisely summarized the key new guideline recommendations. First, he notes again that prostate cancer continues to be the most common solid malignancy identified in men (286K new cases) and 2nd leading cause of cancer death (34,760 cases).
While multiple studies (ERSPC and others) have demonstrated the benefit of early detection and treatment of clinically significant prostate cancer (csPCa), screening is a preference sensitive decision that may have many potential downstream implications.
As a brief background, he provided some understanding of the history of the Early Detection guidelines and the process used to develop the guidelines.
- AUA Early Detection of Prostate Cancer (2013, 2018) focused primarily on screening and age-based recommendations
- AUA EDPC 2023 provides a completely new set of recommendations
- Refined recommendations for screening
- New recommendations related to biomarkers, MRI and biopsy techniques
- Recommendations were categorized into 4 topics/workgroups
- Screening
- Initial biopsy (elevated PSA, no prior biopsy)
- Repeat biopsy (prior negative biopsy)
- Biopsy technique
The methodology is rigorous.

Once the lit search is done, each working group only developed recommendations from the available data – they did not bring in data from the outside.

Key take-home points are broken down below.
He made a point to take into account the use of the word “May” and “Should” – where “Should” is a much stronger recommendation than “May.”
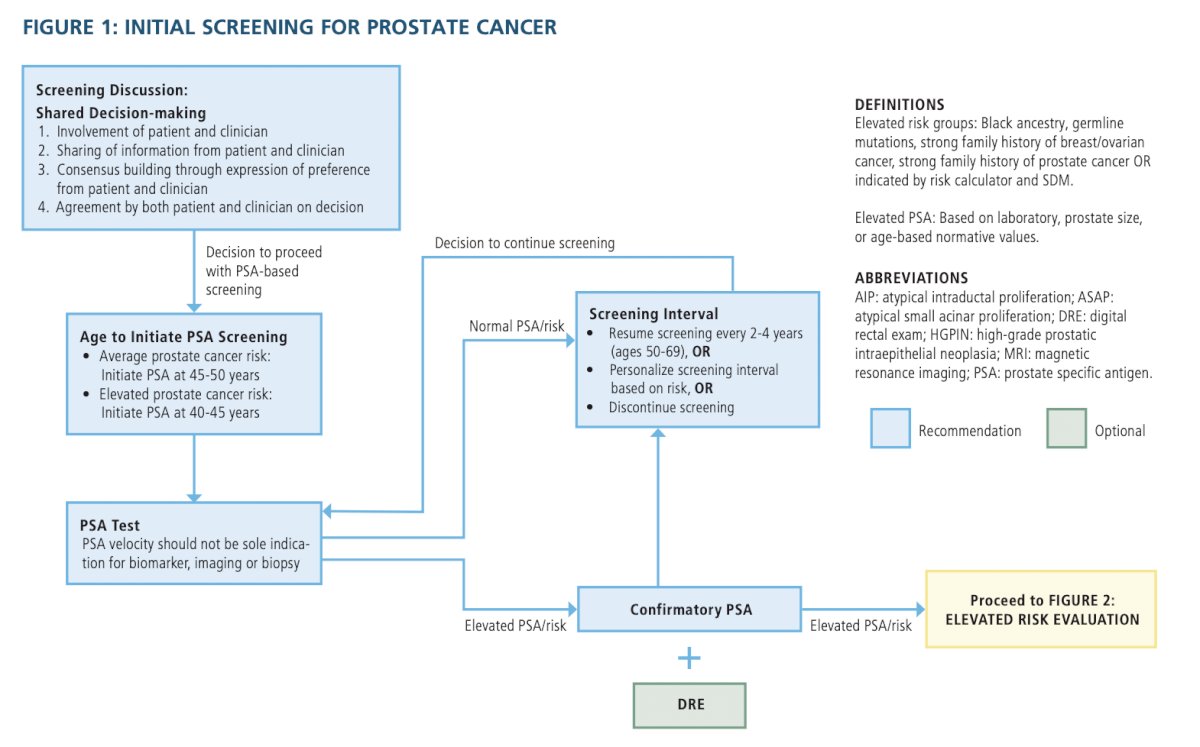
SCREENING- Clinicians should engage in shared decision-making (SDM) with people for whom prostate cancer screening would be appropriate and proceed based on a person’s values and preferences. (Clinical Principle)
- When screening for prostate cancer, clinicians should use PSA as the first screening test. (Strong Recommendation; Evidence Level: Grade A)
- They evaluated additional tests, but none exceeded PSA as an initial screening test
- For people with a newly elevated PSA, clinicians should repeat the PSA prior to a secondary biomarker, imaging, or biopsy. (Expert Opinion)
- Clinicians may use digital rectal exam (DRE) alongside PSA to establish risk of clinically significant prostate cancer. (Conditional Recommendation; Evidence Level: Grade C)
- DRE is still important for staging, but not necessary for initial screening
- For people undergoing prostate cancer screening, clinicians should not use PSA velocity as the sole indication for a secondary biomarker, imaging, or biopsy. (Strong Recommendation; Evidence Level: Grade B)
- Can be used in context of other data, but not by itself
- Clinicians may begin prostate cancer screening and offer a baseline PSA test to people between ages 45 to 50 years. (Conditional Recommendation; Evidence Level: Grade B)
- This is new!
- Clinicians should offer prostate cancer screening beginning at age 40 to 45 years for people at increased risk of developing prostate cancer based on the following factors: Black ancestry, germline mutations, strong family history of prostate cancer. (Strong Recommendation; Evidence Level: Grade B)
- This is new!
- Clinicians should offer regular prostate cancer screening every 2 to 4 years to people aged 50 to 69 years. (Strong Recommendation; Evidence Level: Grade A)
- Note the adjusted time frame
- Clinicians may personalize the re-screening interval, or decide to discontinue screening, based on patient preference, age, PSA, prostate cancer risk, life expectancy, and general health following SDM. (Conditional Recommendation; Evidence Level: Grade B)
- Clinicians and patients may use validated risk calculators to inform the SDM process regarding prostate biopsy. (Conditional Recommendation; Evidence Level: Grade B)
- There are multiple calculators, but they can be used to inform patients about risk of csPCa to determine need for prostate biopsy
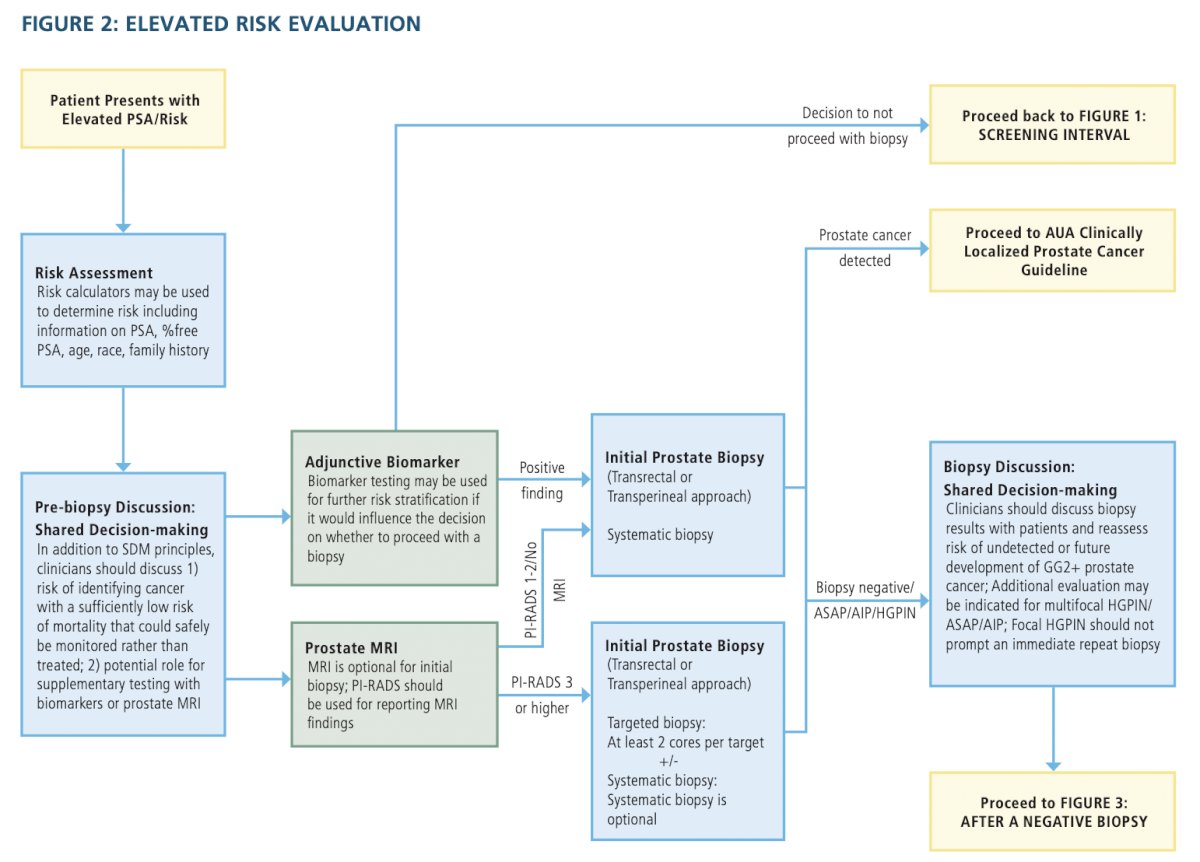
- When the risk of clinically significant prostate cancer is sufficiently low based on available clinical, laboratory, and imaging data, clinicians and patients may forgo near-term prostate biopsy. (Clinical Principle)
- Clinicians should inform patients undergoing a prostate biopsy that there is a risk of identifying a cancer with a sufficiently low risk of mortality that could safely be monitored with active surveillance (AS) rather than treated. (Clinical Principle)
- Patients should be aware of the possibility and implications of a low risk GG1 PCa diagnosis
- Clinicians may use magnetic resonance imaging (MRI) prior to initial biopsy to increase the detection of Grade Group (GG) 2+ prostate cancer. (Conditional Recommendation; Evidence Level: Grade B)
- Radiologists should utilize PI-RADS in the reporting of multi-parametric MRI (mpMRI) imaging. (Moderate Recommendation; Evidence Level: Grade C)
- For biopsy-naïve patients who have a suspicious lesion on MRI, clinicians should perform targeted biopsies of the suspicious lesion and may also perform a systematic template biopsy. (Moderate Recommendation [targeted biopsies]/Conditional Recommendation [systematic template biopsy]; Evidence Level: Grade C)
- Targeted +/- systematic biopsies
- For patients with both an absence of suspicious findings on MRI and an elevated risk for GG2+ prostate cancer, clinicians should proceed with a systematic biopsy. (Moderate Recommendation; Evidence Level: Grade C)
- Clinicians may use adjunctive urine or serum markers when further risk stratification would influence the decision regarding whether to proceed with biopsy. (Conditional Recommendation; Evidence Level: Grade C)
- For patients with a PSA > 50 ng/mL and no clinical concerns for infection or other cause for increased PSA (e.g., recent prostate instrumentation), clinicians may omit a prostate biopsy in cases where biopsy poses significant risk or where the need for prostate cancer treatment is urgent (e.g., impending spinal cord compression). (Expert Opinion)
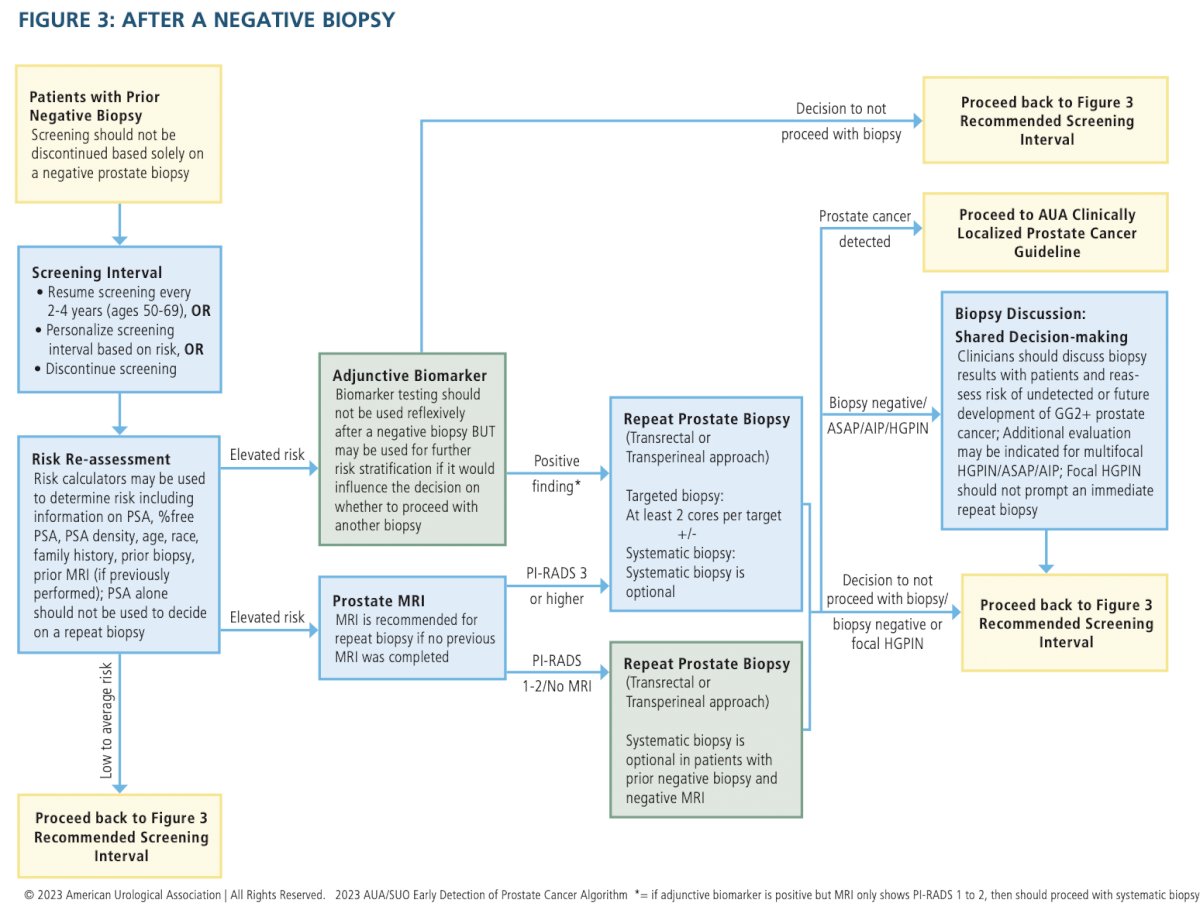
- Clinicians should communicate with patients following biopsy to review biopsy results, reassess risk of undetected or future development of GG2+ disease, and mutually decide whether to discontinue screening, continue screening, or perform adjunctive testing for early reassessment of risk. (Clinical Principle)
- Clinicians should not discontinue prostate cancer screening based solely on a negative prostate biopsy. (Strong Recommendation; Evidence Level: Grade C)
- After a negative biopsy, clinicians should not solely use a PSA threshold to decide whether to repeat the biopsy. (Strong Recommendation; Evidence Level: Grade B)
- PSA can be followed, but should not be the main trigger for repeat biopsy.
- If the clinician and patient decide to continue screening after a negative biopsy, clinicians should re-evaluate the patient within the normal screening interval (two to four years) or sooner, depending on risk of clinically significant prostate cancer and life expectancy. (Clinical Principle)
- At the time of re-evaluation after negative biopsy, clinicians should use a risk assessment tool that incorporates the protective effect of prior negative biopsy. (Strong Recommendation; Evidence Level: Grade B)
- Nomograms and calculators in this setting are different than biopsy naive
- After a negative initial biopsy in patients with low probability for harboring GG2+ prostate cancer, clinicians should not reflexively perform biomarker testing. (Clinical Principle)
- After a negative biopsy, clinicians may use blood, urine, or tissue-based biomarkers selectively for further risk stratification if results are likely to influence the decision regarding repeat biopsy or otherwise substantively change the patient’s management. (Conditional Recommendation; Evidence Level: Grade C)
- Mainly to risk stratify IF it will change management
- In patients with focal (one core) high-grade prostatic intraepithelial neoplasia (HGPIN) on biopsy, clinicians should not perform immediate repeat biopsy. (Moderate Recommendation; Evidence Level: Grade C)
- In patients undergoing repeat biopsy with no prior prostate MRI, clinicians should obtain a prostate MRI prior to biopsy. (Strong Recommendation; Evidence Level: Grade C)
- If they haven’t had one already, now is the time to get it.
- In patients with indications for a repeat biopsy who do not have a suspicious lesion on MRI, clinicians may proceed with a systematic biopsy. (Conditional Recommendation; Evidence Level: Grade B)
- In patients undergoing repeat biopsy and who have a suspicious lesion on MRI, clinicians should perform targeted biopsies of the suspicious lesion and may also perform a systematic template biopsy. (Moderate Recommendation [targeted biopsies]/Conditional Recommendation [systematic template biopsy]; Evidence Level: Grade C)
- Targeted +/- systematic
- Clinicians may use software registration of MRI and ultrasound images during fusion biopsy, when available. (Expert Opinion)
- Clinicians should obtain at least two needle biopsy cores per target in patients with suspicious prostate lesion(s) on MRI. (Moderate Recommendation; Evidence Level: Grade C)
- Adequate sampling is at least two needle biopsy cores per target
- Clinicians may use either a transrectal or transperineal biopsy route when performing a biopsy. (Conditional Recommendation; Evidence Level: Grade C)
- At this time, they did not feel the data supported one or the other strongly.
He acknowledged that there are many areas where the evidence is still lacking or immature – and likely will need to be updated on later iterations of the guidelines.
Presented by: John Thomas Wei, MD, Professor of Urology, University of Michigan, Ann Arbor, MI
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023


