(UroToday.com) In this presentation at the American Urologic Association (AUA) annual meeting, Dr. Kelly Stratton presents the initial results of the ABC trial, an open-label Phase Ib trial of BCG therapy in conjunction with avelumab for patients with BCG unresponsive non-muscle invasive bladder cancer (NMIBC). From a safety standpoint, they found that induction therapy with combined BCG and avelumab was safe and well-tolerated.
As the authors note, the optimal management of Bacillus Calmette-Guerin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) remains unclear. Options include intravesical therapies (gem/doce), systemic therapies (pembrolizumab), and ultimately, the gold standard, early radical cystectomy. Given the limited treatment options, the FDA suggested single-arm studies may provide evidence of treatment efficacy. Immunotherapy responses in patients with metastatic urothelial carcinoma have created interest in their use in the NMIBC setting. Pembrolizumab has already been approved as a monotherapy in this situation. However, combination therapy with BCG for patients with BCG-unresponsive NMIBC remains of interest – potentially re-activating and increase response to BCG. But, the optimal dosing of immunotherapy in combination with BCG treatment is not clear. This study evaluates the safety and tolerability of a novel intense weekly dosing regimen of avelumab during BCG induction for patients with BCG-unresponsive NMIBC.
They opted for this regimen based on data regarding the pharmacokinetics of the clearance of avelumab and pembrolizumab, in addition to the data/tolerability of the dosing in the JAVELIN Lung 100 trial (arm C)
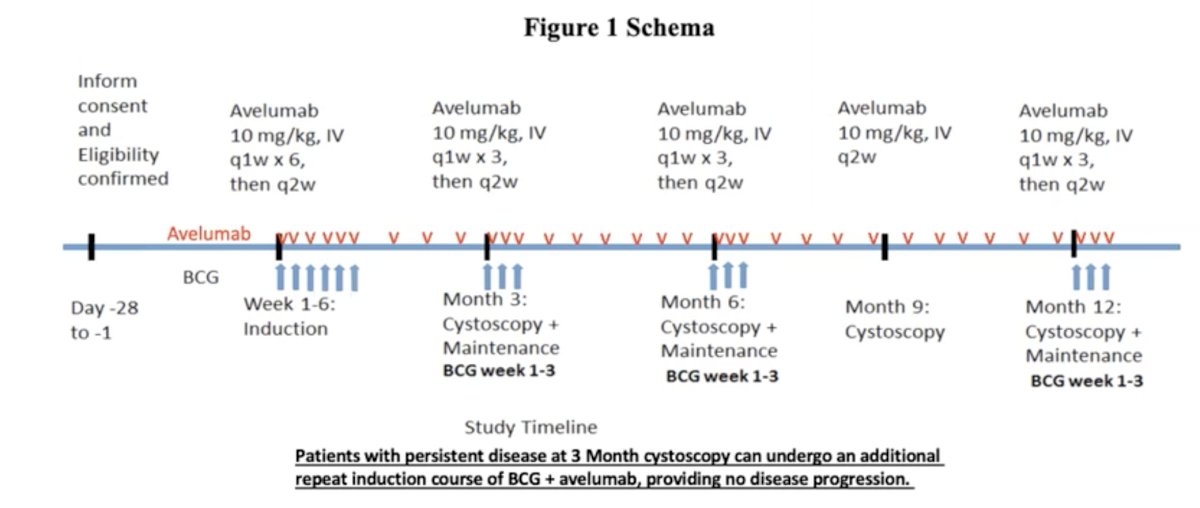
The ABC trial (NCT03892642) was an open-label Phase Ib trial of adults with a history of NMIBC and prior BCG therapy. Following tumor resection, patients underwent a 6-week BCG induction with weekly avelumab infusion (10 mg/kg IV) followed by BCG maintenance therapy with weekly avelumab for 12 months. In between BCG treatments, bi-weekly avelumab infusion (10 mg/kg IV) was given instead of weekly doses. The primary endpoint was the completion of a full induction course, five out of six treatments with BCG and avelumab, within eight weeks of treatment initiation. The secondary endpoint was the completion of 6 months of maintenance treatment. The goal of this study was not treatment efficacy.
Full dosage schedule is listed below:
The trial enrolled 18 patients, of which 4 were Female and 14 Male. There were 2 Black, 16 White, and no Hispanic or Latino patients. Median age was 69.9 ± 9.3 years (range, 53-84). The majority of patients (9) had T1 stage tumors, 5 patients had Ta, and 4 patients had Tis.
Patients had received prior BCG induction and maintenance doses, two had even received intravesical chemotherapy.
Looking at tolerability, a total of 15/18 (83%) patients were able to complete induction therapy with 16 receiving at least 5 BCG treatments and 15 receiving at least 5 avelumab treatments. This exceeded the 60% anticipated completion rate.
At the three-month cystoscopy, 13/15 (87%) patients remained on treatment, 10 without evidence of cancer and 3 with persistent disease (2 CIS, 1 Ta).
5 patients have completed 12 months of therapy, and all 5 patients had CR.
2 patients remain on treatment at this time.
Looking at safety, no drug-attributable grade 4 or 5 AE’s were observed. All patients who discontinued treatment were included in the safety analysis. During induction therapy 2 (11%) patients had probable drug-related grade 3 adverse events which were: infusion-related reaction (avelumab) and sepsis (BCG). One patient developed Grade 3 adverse event (patient had myasthenia gravis) during maintenance therapy. Follow-up remains ongoing.
Based on this initial report of the ABC trial, in BCG-unresponsive NMIBC patients, induction therapy with combined BCG and avelumab was safe and well-tolerated. This study supports continued efforts to evaluate the optimal dosing regimen to synergize BCG and immunotherapy.
Presented by: Kelly Stratton, MD, FACS, assistant professor of Urologic Oncology in the Oklahoma University Department of UrologyWritten by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.


