(UroToday.com) Prostate-specific membrane antigen (PSMA) is selectively overexpressed in most advanced prostate cancer tumors with limited expression in other organs (including celery and lacrimal glands, renal tubules, and small intestines).
Sequential prospective clinical trials of anti-PSMA mAb J591 and small molecule PSMA-617 radiolabelled with the beta emitter 177 LU, demonstrated tolerability with accurate targeting in an unselected patient population with metastatic castrate-resistant prostate cancer. It also has shown a dose-response relationship with prostate-specific antigen (PSA) and overall survival.
Figure 1 demonstrates the differences between the mAb and Ligand, and Figure 2 demonstrates the differences between antibody, minibody, and small molecule.
Figure 1 – Differences between mAb and Ligand: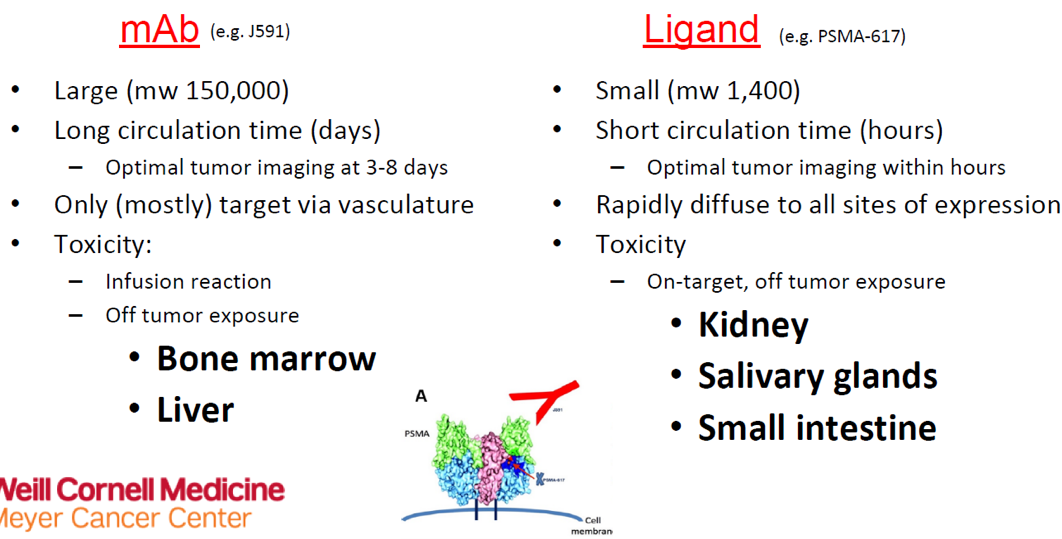
Figure 2 – Differences between antibody, minibody, and small molecule:
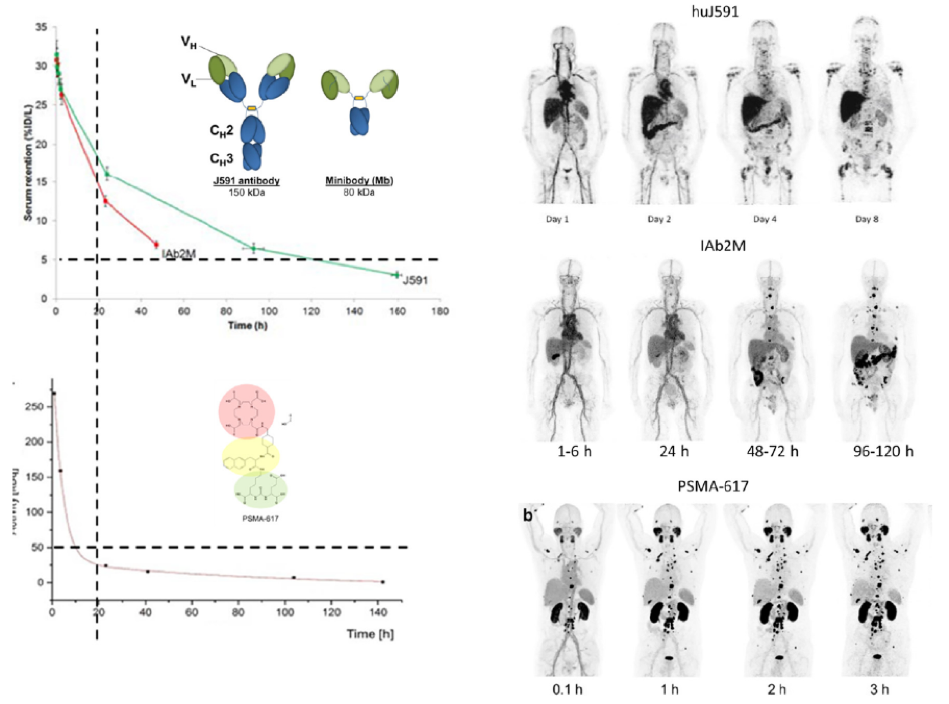
The authors hypothesized that because of the difference in physical properties that lead to differences in kinetics and biodistribution, the adverse events observed following LU-J591 and Lu-PSMA-617 will most likely be different.
In the presented study, the authors extracted all adverse effects data, PSA changes, and overall survival information from the prospective clinical trial data, which enrolled patients with metastatic castrate-resistant prostate cancer, who were treated with PSMA-targeted lutetium-177.
The primary endpoint was the comparison of selected treatment-emergent adverse events between J591 and Lu-PSMA-617. Exploratory endpoints included PSA decline and overall survival. Baseline characteristics are shown in Table 1.
Table 1- Baseline characteristics: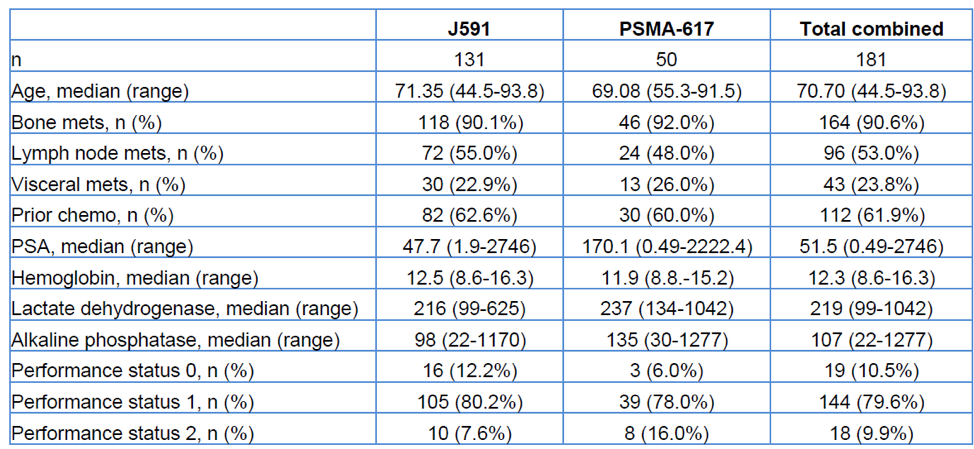
The results demonstrated in Table 2 show that there were more hematological treatment-emergent adverse events with mAb targeting, while there were more none-hematological treatment-emergent adverse events with ligand treatment, even after controlling for dose.
Table 2 – Comparison of adverse events:
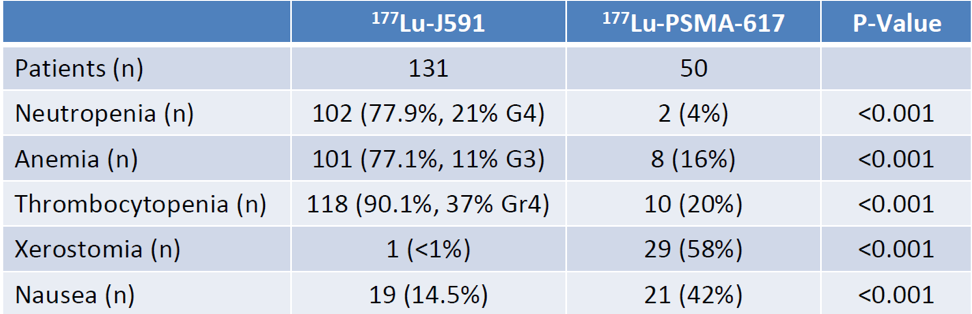
The results also demonstrated there was more than 30% PSA decline with PSMA-617 (adjusted OR 6.37, 95% CI 2.97-14.46), compared to J591.
Additionally, the unadjusted median overall survival with PSMA-617 was shorter than for J591 (14.2 vs. 19.6 months). On multivariable analysis, significant associations with overall survival were seen with:
- A higher administered dose
- CALGB (Halabi) prognostic group
- Subsequent receipt of FDA-approved life-prolonging therapies (docetaxel, sipuleucel-T, cabazitaxel, abiraterone, enzalutamide, radium-223)
The limitations of this study include the fact that there was a lot of variability in the patients enrolled, as they were enrolled between 2002 and 2019, with many changes occurring along the way. Additionally, several of the trials were dose-escalation studies, affecting the dose patients received. Lastly, a different version of the adverse events grading system was used through the years (CTCAE v3 used in early studies vs. CTCAE v4 used in later studies).
The authors concluded that as they had originally hypothesized:
- PSMA targeted 177Lu delivered via intact antibody J591 is associated with more hematologic toxicity than with small molecule delivery
- PSMA targeted 177Lu delivered via small molecule PSMA-617 is associated with more non-hematological toxicity than antibody delivery
- 177Lu-PSMA-617 was shown to be associated with more PSA decline than Lu-J591, but no difference in overall survival was evident on multivariable analysis
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Twitter: @GoldbergHanan, at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27-28, 2020.
Related Content:
Read: Preparing Your Practice for the New Era of Theranostics


