(UroToday.com) The guidelines for microhematuria were formulated by a multidisciplinary panel with representations from the American Urological Association (AUA), Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU), and American College of Obstetricians and Gynecologists (ACOG), as well as Bladder Cancer Advocacy Network (BCAN) patient advocate.
The guidelines were based on systematic review search dates between January 2010 until December 2019. The evidence base includes five systematic reviews and 91 primary literature studies.
Hematuria is one of the most common urological diagnoses, with over 25% of urological evaluations. The prevalence of microscopic hematuria from screening healthy volunteers is approximately 6.5%, with ranges between 2.4 and 31.1%, depending on the specific population evaluated.
The possible urological etiologies for haematuria include:
- malignancy in approximately 3% of cases
- infection
- Inflammation
- Stone disease
- benign prostatic hyperplasia
- congenital or acquired anatomic abnormalities
There is significant variability across the current guidelines and consensus statements regarding the evaluation of microscopic haematuria, whether it should entail cystoscopy and upper tract imaging.
It is known that aside from the conflicting guidelines regarding the evaluation of microscopic hematuria, there is an overall low yield of the evaluation with malignancy diagnosed in only 3% of the cases, with less than 1% diagnosed in patients without any kind of risk factors, and more than 10% diagnosis in patients with multiple risk factors. Additionally, the evaluation also has potential harms, which include risks to the patient and cost to the health system. Moreover, currently, there is poor adherence to the existing guidelines.
The aim of the 2020 AUA guidelines regarding microscopic haematuria is to provide a risk-stratified approach to hematuria evaluation based on the patient’s risk of harboring a urinary tract cancer and concordant with the patients’ values.
Microhematuria is defined as three or above red blood cells per high power field on microscopic evaluation of a single, properly collected urine specimen (Evidence level grade C). Clinicians should not define microhematuria by a positive dipstick testing alone. Positive dipstick test trace blood should prompt formal microscopic evaluation of the urine. In patients diagnosed with gynecological or non-malignant genitourinary sources of microhematuria, clinicians should repeat urinalysis following the resolution of the gynecological or non-malignant genitourinary cause. If microhematuria persists or the etiology cannot be identified, clinicians should perform a risk-based urological evaluation.
If the hematuria is attributed to a urinary tract infection, clinicians should obtain a urinalysis with microscopic evaluation following treatment of the infection to ensure that hematuria has been resolved (Evidence level grade C ).
Following the initial evaluation, clinicians should categorize patience presenting with microhematuria either as low, intermediate, or high risk for genitourinary malignancy based on the following tables (Table 1).
Table 1-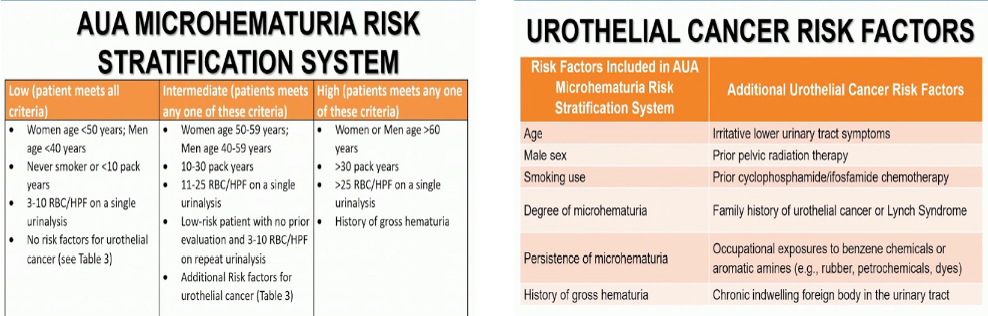
Specifically, in patients with low risk of malignancy, the clinician should engage in a shared decision-making process with the patient in an attempt to decide between repeating the urinalysis within six months or proceeding with cystoscopy and renal ultrasound. If these low-risk patients initially elect not to undergo cystoscopy or upper tract imaging and are found to have microhematuria on repeat urine testing, they should be reclassified as intermediate or high-risk. In these patients, upper tract imaging and cystoscopy should be done in accordance with recommendations for these extra risk strata.
In intermediate-risk patients, clinicians should perform cystoscopy and renal ultrasound.
Lastly, in high-risk patients, cystoscopy and upper tract imaging should be performed as well. Specifically, in high-risk patients, if there is no contraindication to its use, clinicians should perform multiphasic CT urography. If there is a contraindication to CT urography, clinicians may utilize MRU. If there are contraindications to both CTU and MRU, retrograde paleography in conjunction with non-contrast axial imaging or renal ultrasound should be done.
No urinary markers, including urine cytology, should be used in the initial evaluation of patients with microhematuria. Urine cytology may be obtained for patients with persistent microhematuria after negative workup, who also have irritative voiding symptoms or risk factors for carcinoma-in-situ.
In patients with a negative workup, clinicians may obtain repeat urinalysis within 12 months. The patient with a prior negative workup and subsequent negative urinalysis clinicians may discontinue further evaluation for microhematuria.
If patients with a prior negative hematuria evaluation have persistent or recurrent microhematuria at the time of repeat urinalysis, clinicians should engage in shared decision making regarding the need for additional evaluation. Patients with prior negative hematuria evaluation, who developed gross hematuria, a significant increase in the degree of their microhematuria, or new urologic symptoms, the clinician should initiate further evaluation.
The panel does acknowledge several notable areas where there are considerable gaps in knowledge. These present opportunities for further investigation to enhance care. These include:
- automated instrumentation
- Validation of risk groups
- lower radiation imaging
- enhanced cystoscopy
- urinary biomarkers
- follow up procedures
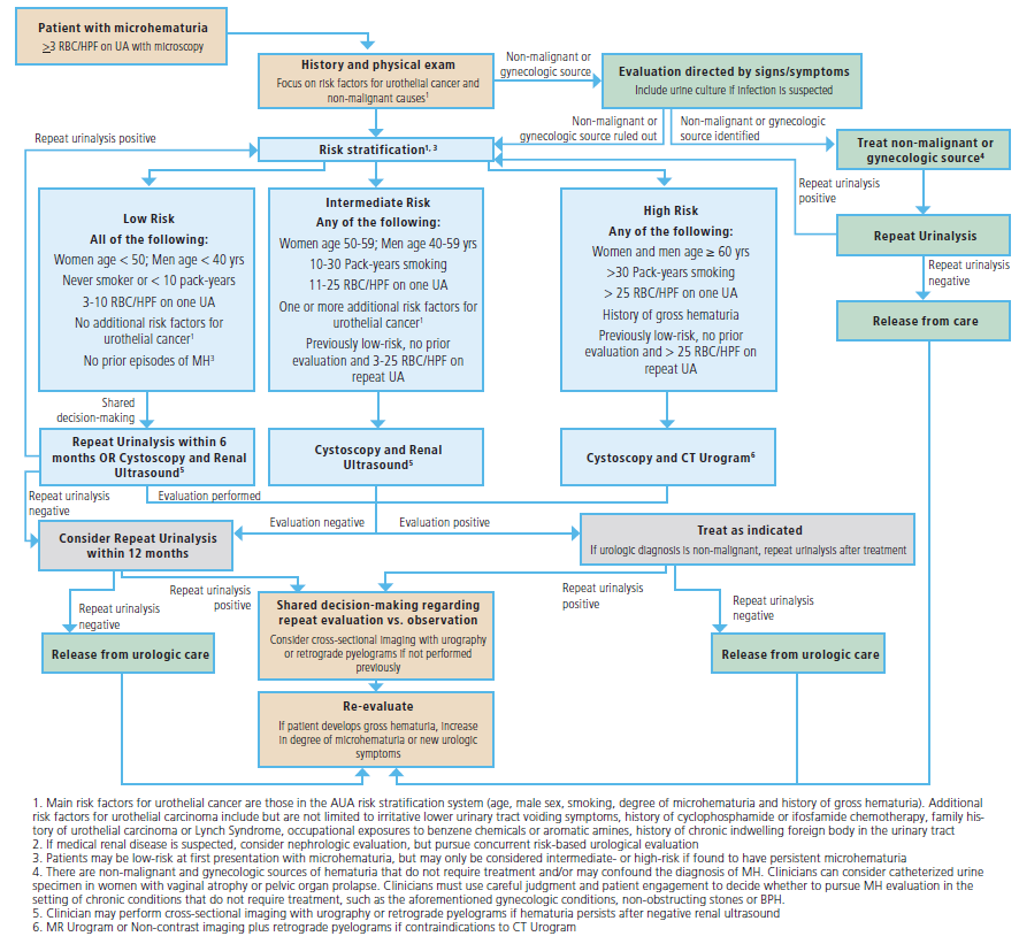
The algorithm for the AUA microhematuria guidelines is depicted in Figure 1.
Figure 1– Microscopic hematuria AUA algorithm guidelines:
Presented by:
Daniel Barocas MD, MPH, FACS, Associate Professor and Vice-Chair, Department of Urology, Vanderbilt University
Stephen A. Boorjian, Carl Rosen Professor of Urology, Vice-Chair of Research for the Department of Urology, and the Director of the Urologic Oncology Fellowship at Mayo Clinic, Rochester, MN
Written By: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020Related Content:
Leading Organizations Release Joint Clinical Guideline for Diagnosis and Evaluation of Microhematuria


