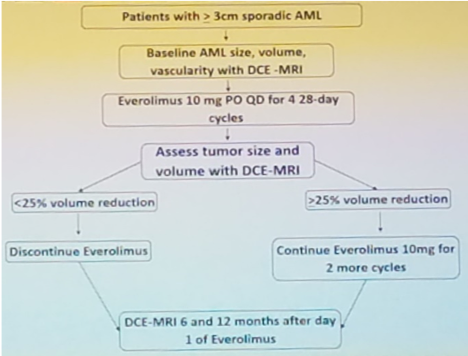
The authors prospectively enrolled eligible patients who presented with sporadic AMLs ≥ 3cm in diameter without prior treatment and were eligible for surgical or percutaneous access AML treatment. Each patient participated in a baseline dynamic contrast-enhanced MRI (DCE-MRI) in which AML diameter, volume, and vascularity were noted. Patients then began treatment with 10mg of EVE PO daily and continued this treatment for 4, 28- day cycles. If patients were unable to tolerate the high dosage of EVE, they were granted at most 2 dosage reductions to 5mg QD and 5mg QOD, respectively. At the end of the 4th cycle, patients underwent another DCE-MRI noting any changes in AML volume. If AML volume decreased by 25%, EVE was discontinued; if AML volume decreased by ≥25%, EVE was continued for an additional 2 cycles. Two more DCE-MRI were completed at 6 and 12 months after the start of the study. Below is an image from Dr. Brian Kadow’s presentation displaying the trial design. The primary outcomes for the study were efficacy and tolerability of EVE. In cases which toxicity and adverse events were of concern, the patient was discontinued from the trial due to the treatment of benign growths.
Overall, 20 patients from 5 different institutions were recruited with a mean AML volume of 47.3cc. Of these participants, 55% (11/20) completed 4 cycles and 35% (7/20) completed 6 cycles, with a median of 3.4 cycles of EVE. Efficacy was determined at 18 patients with 55.6% (10/18) of participants experiencing a ≥25% decrease in AML volume after 4 cycles. The mean AML volume decreased 55.5% and non-respondent patients had an average decrease in volume of 11.8% after 4 cycles. With 20 patients enrolled, an early toxicity stopping point was reached with 20% (4/20) of patients withdrawn due to pre-defined toxicities. Despite 30% (6/20) of patients reducing their dosage, patients suffered from grade 3 oral mucositis, grade 2 hyperglycemia, and grade 2 and 1 pneumonitis. Another 40% (8/20) of patients decided to discontinue the study due to personal preferences and 95% (19/20) of patients experienced ≥1 grade 2 adverse effects. Below is the data correlating to the adverse effects experienced and amount of occurrence.

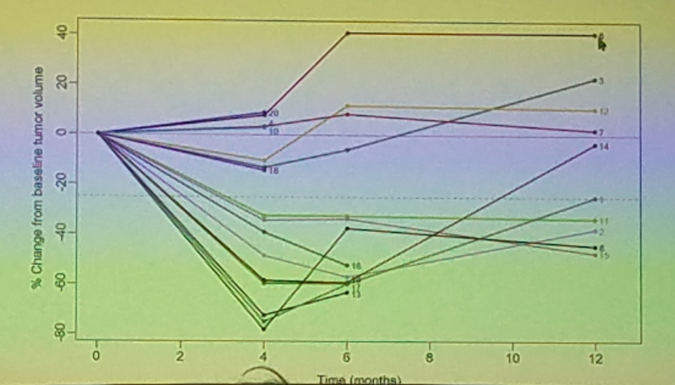
Dr. Brian Kadow concluded that EVE was effective in its ability to decrease AML volume by at least 25% in most patients with sporadic AMLs. However, due to the benign condition, a large portion of patients were unenrolled from the study due to personal preferences (40%). Additionally, 20% of patients were unenrolled due to pre-defined toxicities. The results of this study highlight the possibility of future treatment of large or complex AMLs with EVE. Upon questioning, AML growth following discontinuation of EVE was addressed. Dr. Brian Kadow noted that the 6 and 12-month DCE-MRIs, on average, showed an increase in AML volume. This trend can be seen in the image below from Dr. Brian Kadow’s presentation.
Presented by: Brian Kadow, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Philadelphia, PA
Co-authors: Daniel Geynisman, Brian Such, Stephen Boorjian, Surena Matin, Edward Rampersaud, Daniel McClean, Barton Milestone, Elizabeth Plimack, Matthew Zibelman, Alexander Kutikov, Marc Smaldone, David Chen, Rosalia Viterbo, Shreyas Joshi, Richard Greenberg, Eric Ross, Lois Malizzia, Thomas McGowan, Robert Uzzo
Written by: Christina Kosmala, (Department of Urology, University of California-Irvine) at the American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois


