(UroToday.com) The 2022 ASTRO annual meeting featured a prostate cancer session, including a presentation by Dr. Bradley DePaoli discussing toxicity outcomes at 18 months for Cs-131 low-dose-rate (LDR) prostate brachytherapy compared to I-125 and Pd-103. LDR prostate brachytherapy is an established component of definitive radiotherapy options for localized prostate cancer. As the utility of LDR brachytherapy has evolved, the focus has shifted to improving the side effect profile while maintaining oncologic outcomes. Most prior efficacy and toxicity data were gathered using I-125 and Pd-103, but due to the shorter half-life and slightly higher average energy of Cs-131, several institutions have adopted its use in an attempt to improve outcomes and decrease toxicity. The objective of this study by Dr. DePaoli and colleagues was to report the toxicity outcomes up to 18 months after Cs-131 LDR brachytherapy, and compare outcomes to their experience with I-125 and Pd-103.
A retrospective review was performed on the first 50 patients treated with Cs-131 LDR brachytherapy for localized prostate cancer. Patients receiving either a full or partial implant were included. Prescription doses of 100 Gy and 80 Gy were utilized, respectively. Patients receiving partial implants generally received 45 Gy external beam radiotherapy starting 6-8 weeks after implant. Data regarding AUA prostate symptom score, sexual health inventory for men (SHIM) score, and RTOG gastrointestinal toxicity score were assessed pretreatment, at 1 month, and at each subsequent follow up. The data from Cs-131 LDR brachytherapy was compared to cohorts of the last 50 patients treated I-125 and the last 50 patients treated with Pd-103, to standard doses. Descriptive statistics were used to summarize variables. A linear mixed model was used to compare means and to identify variables as prognostic for toxicity (p ≤ 0.05).
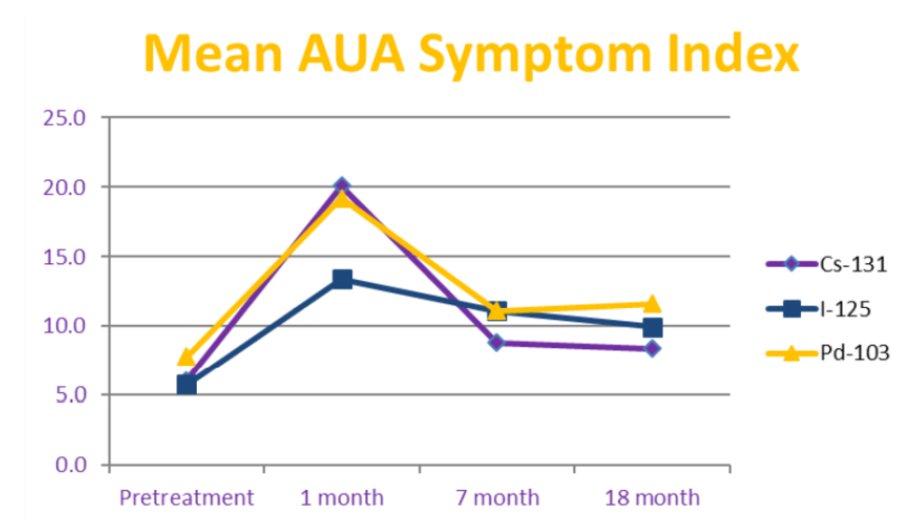
The mean AUA scores prior to brachytherapy were similar for patients in all groups (p = 1.00), while at 1-month post-treatment, mean AUA score was significantly higher for patients treated with Cs-131 and Pd-103 compared to I-125 (p < 0.001). Mean AUA scores were not significantly different at longer follow-up (p >0.50):
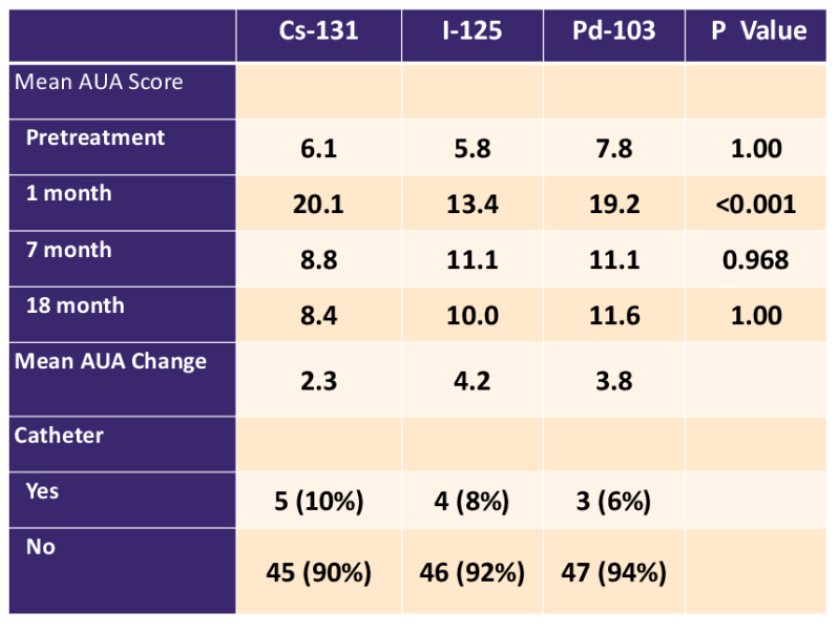
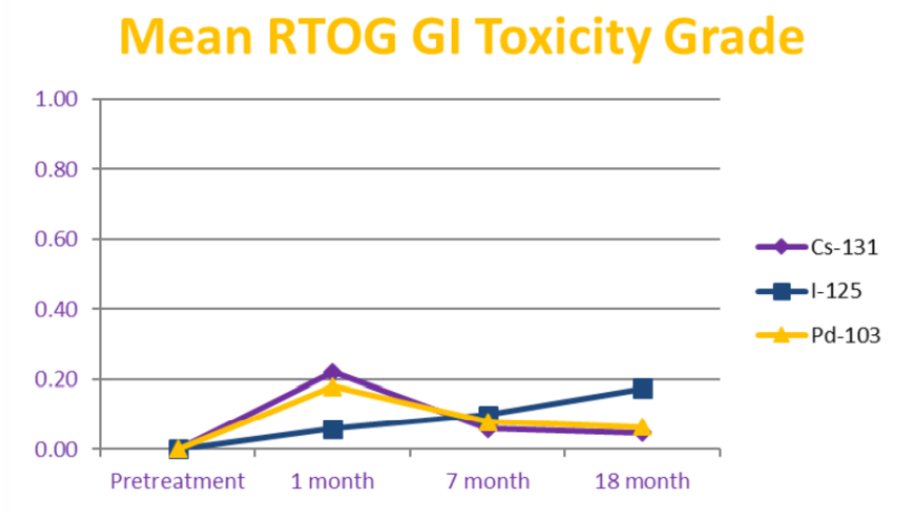
The need for a catheter post-brachytherapy occurred in 8% of patients and was not associated with type of isotope on multivariable analysis. Prostate volume, numbers of seeds, and prostate D90% were significant predictors for the need for a catheter on multivariable analysis. The use of ADT and the total activity were significant predictors on multivariable analysis for a decrease in SHIM score over time. The type of isotope used did not predict for erectile dysfunction as measured by SHIM score on multivariable analysis. Overall rates of GI toxicity were low with only 3 patients developing RTOG grade 2 toxicity or greater without any obvious difference between isotopes:
Dr. DePaoli concluded his presentation discussing toxicity outcomes at 18 months for Cs-131 low-dose-rate prostate brachytherapy compared to I-125 and Pd-103 with the following take home messages:
- In patients treated with Cs-131 or Pd-103 LDR brachytherapy, acute genitourinary side effects peaked higher at 1 month than with I-125, but rapidly decreased to similar levels with longer follow-up
- The type of source used did not predict for need for catheterization, erectile dysfunction, or gastrointestinal toxicity at any time point
- Prostate LDR brachytherapy can be delivered with any of these 3 isotopes with similar expectations of low levels of toxicity at 18 months
Presented by: Bradley DePaoli, MD, Medical University of South Carolina - Hollings Cancer Center, Charleston, SC
Co-Authors: S. M. McVorran2, M. J. Case3, S. Khaleghi3, H. Li3, and D. T. Marshall1; 1Medical University of South Carolina - Hollings Cancer Center, Charleston, SC, 2H. Lee Moffitt Cancer Center and Research Institute, Department of Radiation Oncology, Tampa, FL, 3Medical University of South Carolina, Charleston, SC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.


