(UroToday.com) The 2022 ASTRO annual meeting featured a Presidential Symposium session on artificial intelligence opportunities in today’s patient’s journey, including a presentation by Dr. Osama Mohamad discussing the state of the art in digital pathology and artificial intelligence in prostate cancer. Dr. Mohamad notes that traditional histopathology includes slide preparation microscopic analysis slide storage, whereas digital histopathology includes slide preparation and whole slide imaging analysis slide storage. The promise of computational pathology is as follows:

Prior to discussing artificial intelligence as a prognostic and predictive biomarker in prostate cancer, Dr. Mohamad outlined the difference between prognostic and predictive biomarkers. Prognostic biomarkers estimate recurrence, while predictive biomarkers guide treatment selection:
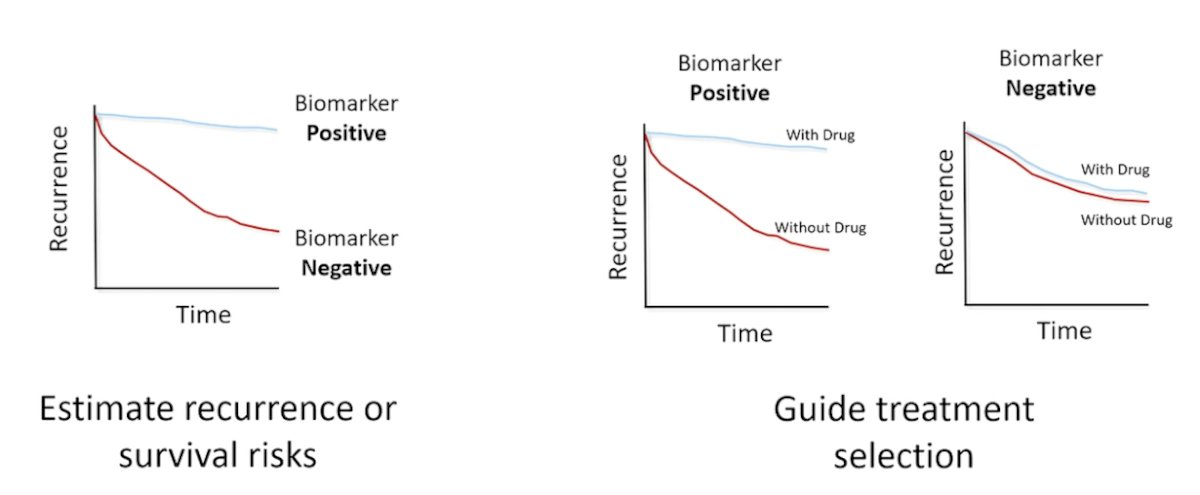
Dr. Mohamad’s group led an initiative to develop accurate and scalable tools to support therapy personalization with prognostic biomarkers. In this key study,1 they were able to personalize prostate cancer therapy by predicting long-term, clinically relevant outcomes using a multimodal deep learning architecture, training models using clinical data and digital histopathology from prostate biopsies. Dr. Mohamad and colleagues trained and validated models using five phase III randomized trials (RTOG 9202, RTOG 9413, RTOG 9910, RTOG 0126, and RTOG 9408), of which histopathological data was available for 5654 of 7764 randomized patients (71%) with a median follow-up of 11.4 years. The multimodal architecture is composed of two parts: a tower stack to parse a variable number of digital histopathology slides and another tower stack to merge the resultant features and predict binary outcomes:

The self-supervised model in the multimodal model was trained to identify whether or not augmented versions of small patches of tissue came from the same original patch, without ever seeing clinical labels. After training, each image patch in the dataset was fed through this model to extract a 128-dimensional feature vector, and the UMAP algorithm was used to cluster and visualize the resultant vectors. Subsequently, a pathologist then asked to interpret the 20 image patches closest to each of the 25 cluster centroids (6 representative patches shown):

Compared to the most common risk-stratification tool-risk groups developed by the National Cancer Center Network (NCCN), these models had superior discriminatory performance across all endpoints, ranging from 9.2% to 14.6% relative improvement in a held-out validation set. This artificial intelligence-based tool (called ArteraAI) improved prognostication over standard tools and allows oncologists to computationally predict the likeliest outcomes of specific patients to determine optimal treatment.
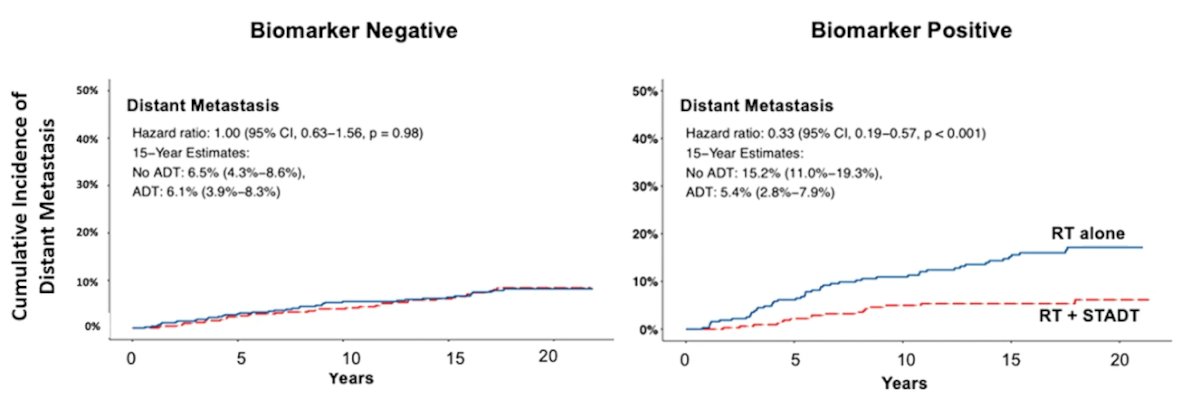
At the 2022 GU ASCO annual meeting Dr. Spratt and colleagues presented data discussing the first predictive biomarker. For this study, pre-treatment biopsy slides were digitized from the same aforementioned five phase III NRG Oncology randomized trials of men receiving radiotherapy with or without ADT. The training set to develop the artificial intelligence-derived predictive biomarker included NRG/RTOG 9202, 9413, 9910, and 0126, and was trained to predict distant metastasis. A multimodal deep learning architecture was developed to learn from both clinicopathologic and digital imaging histopathology data and identify differential outcomes by treatment type. After the model was locked, an independent biostatistician performed validation on NRG/RTOG 9408, a phase III randomized trial of radiotherapy +/- 4 months of ADT:

In patients with artificial intelligence-biomarker positive disease (n = 673, 39%), ADT had a greater benefit compared to radiotherapy alone (HR 0.33, 95% CI 0.19 to 0.57). In the biomarker negative subgroup (n = 1046, 61%), the addition of ADT did not improve outcomes over radiotherapy alone (HR 1.00, 95% CI 0.63 to 1.56). The 15-year distant metastasis rate difference between radiotherapy versus radiotherapy + ADT in the biomarker negative group was 0.4% vs biomarker positive group 9.8%:
Dr. Mohamad notes that there are several challenges of artificial intelligence and digital pathology, noted as follows:
- Issues with datasets (small, mislabeled, biased, artifacts)
- External and prospective validation
- Data and model drifts
- Legal and ethical (biased algorithms)
- Inconsistent reporting
- Regulatory/FDA
- Reimbursement
- Clinical deployment
Dr. Mohamad concluded his presentation discussing the state of the art in digital pathology and AI in prostate cancer with the following take home messages:
- Artificial intelligence and digital pathology play an increasingly important role in the oncology care pathway
- Multiple diagnostic, and predictive models have been developed and some are already approved by the FDA or incorporated into national guidelines
- Further work is needed to unlock the full potential of computational pathology and show its value in the clinic
Presented by: Osama Mohamad, MD, PhD, UCSF Radiation Oncology, San Francisco, CA
References:


