(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Ridvan Demirci discussing the association between PET-based TheraP eligibility and 177Lu-PSMA-617 outcomes in VISION-eligible patients with metastatic castration-resistant prostate cancer (mCRPC). The optimal threshold based on pre-treatment PET for patient selection for LuPSMA is yet to be defined. In the TheraP trial,1 there was a high threshold for PSMA-expression on PSMA PET (at least a lesion with SUVmax >= 20 with all measureable lesions with SUVmax >= 10) and utilized FDG PET to exclude FDG-avid/non-PSMA-avid disease, while the VISION trial2 used only PSMA PET with a lower threshold of PSMA-expression (lesions with uptake > liver) with no measurable lesions with uptake less than the liver:

The stricter criteria in TheraP trial doubled the screening failure rate compared to VISION trial, 28% vs. 12.6%. However, PSA response was higher in TheraP than in VISION. In this study among VISION-eligible patients, Dr. Demirci and colleagues aimed to compare the outcome of those who were TheraP-eligible to TheraP-ineligible. Further, the prognostic value of FDG-PET phenotyping is investigated.
This retrospective cohort study evaluated consecutive VISION-eligible mCRPC patients who received at least one cycle of LuPSMA and had both PSMA- and FDG-PET within one month of each other between June 2022 and January 2023. Patients were blindly classified as TheraP-eligible vs. TheraP-ineligible. The authors compared whole-body semi-quantitative parameters including total tumor volume, SUVmean, and SUVmax, and outcomes including PSA decline of ≥50% relative to baseline (PSA50), PSA-progression-free survival, and overall survival between the groups. Separately, the patients were dichotomized to having dominant FDG-avid disease (uptake greater than the liver in most sites of disease) versus non-FDG-avid disease, and overall survival was compared by Cox-regression.
There were 75 patients, with a median age of 74 years, 89% with an ECOG 0-1, and 28% with visceral disease, included with a median follow-up of 12 months (IQR: 6.5-15):

Overall, 28% (21/75) of patients were assessed as TheraP-ineligible and had lower PSMA-PET SUVmean and SUVmax compared to TheraP-eligible patients, while other PET parameters including PSMA total tumor volume and FDG parameters were not statistically different:

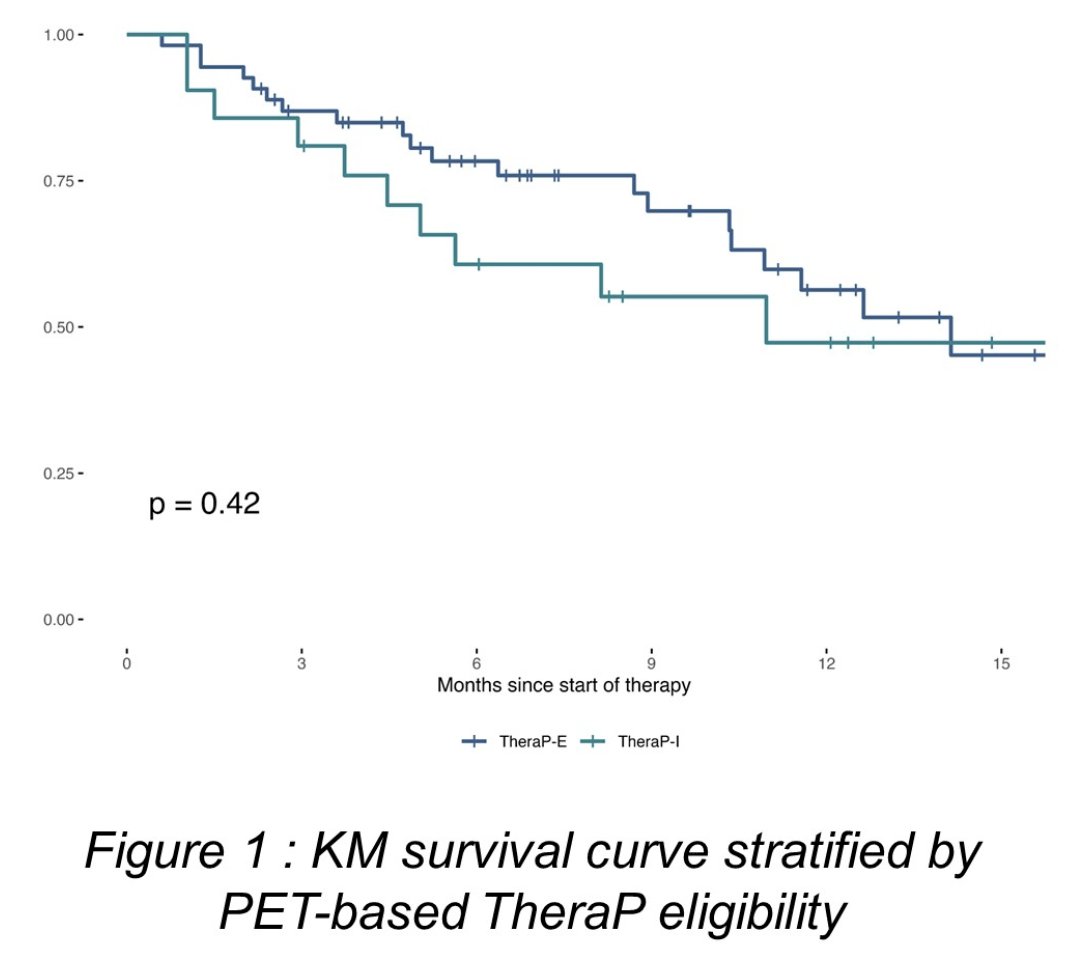
TheraP-ineligible patients had lower PSA50 response (20% vs. 61%, p = 0.002) but PSA-progression-free survival and overall survival were not significantly different compared to TheraP-eligible patients:
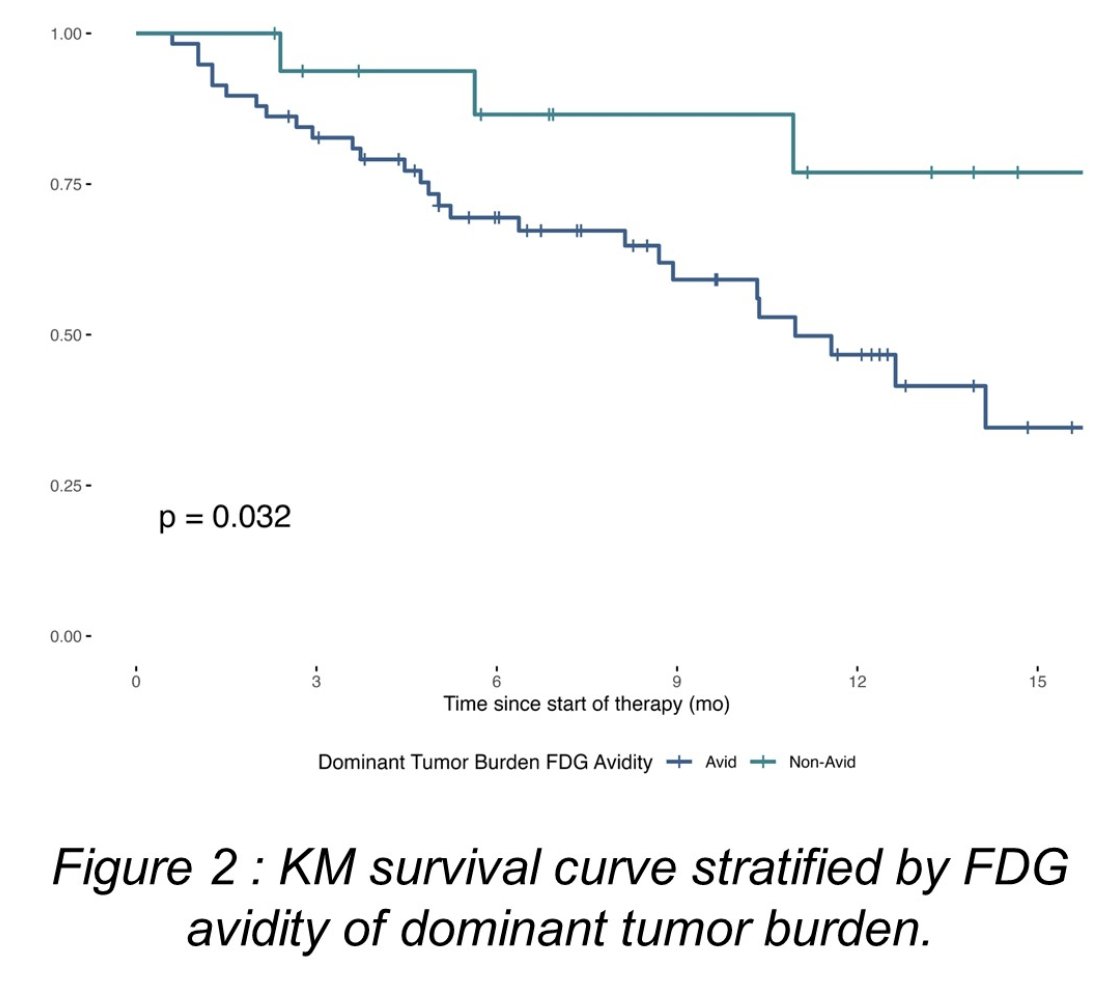
Overall, 80% of patients assessed as FDG-avid which had an increased risk of death compared to non-FDG-avid counterparts (p = 0.032):
Dr. Demirci concluded his presentation by discussing the association between PET-based TheraP eligibility and 177Lu-PSMA-617 outcomes in VISION-eligible patients with mCRPC with the following take-home points:
- In a VISION-eligible population receiving LuPSMA, TheraP imaging-based ineligibility was associated with worse PSA response but with no significant difference in PSA-progression-free survival or overall survival
- Clinical investigation of the more permissive VISION inclusion criteria and the importance of FDG-avid disease using a larger sample size is warranted
Presented by: Ridvan Arda Demirci, University of Washington, Seattle, WA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


