(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Soumaya Labidi discussing clinical outcomes with systemic therapy given after progression on PSMA radioligand therapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
PSMA radioligand therapy is a novel therapeutic modality that improves overall survival in mCRPC.1 However, outcomes for systemic therapy given after progression on PSMA radioligand therapy are not well described. The purpose of this study was to investigate treatment choices and outcomes in patients with mCRPC who experienced disease progression after PSMA radioligand therapy.
Dr. Labidi and colleagues performed a single center retrospective cohort study of patients with mCRPC, who received PSMA radioligand therapy at the Jewish General Hospital, Montreal, Quebec, Canada between 2020 and 2023. Clinical characteristics, PSA50, further lines of therapy post PSMA radioligand therapy, and survival were abstracted from chart review. A log-rank test was used to assess overall survival. Post-PSMA radioligand therapy specific overall survival was defined from the date of first systemic treatment after PSMA radioligand therapy failure to death or last follow up.
This study identified 55 patients who received PSMA radioligand therapy for mCRPC, of which 23 subsequently received another line of therapy (41.8%). The median age was 66.3 years (range: 46-89) and the majority (49.1%) of patients received PSMA radioligand therapy as a third or later line of therapy in mCRPC setting: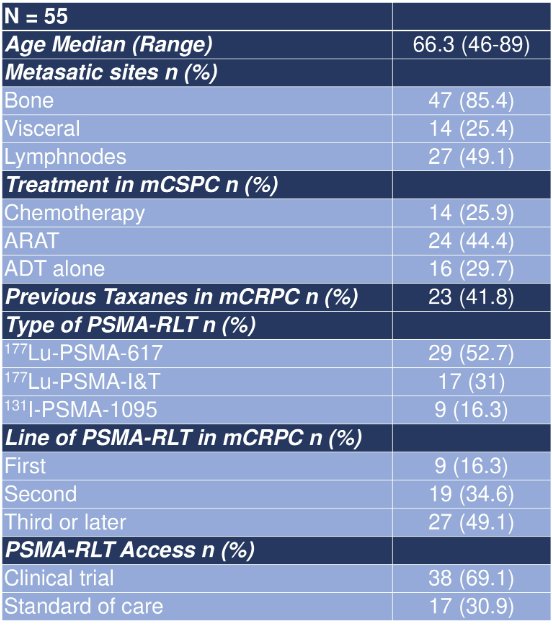
Amongst these patients, 35.7% achieved a PSA50 response to PSMA radioligand therapy. Post-PSMA radioligand therapy systemic therapy most commonly was chemotherapy (n = 10), and PARP inhibitors (n = 3):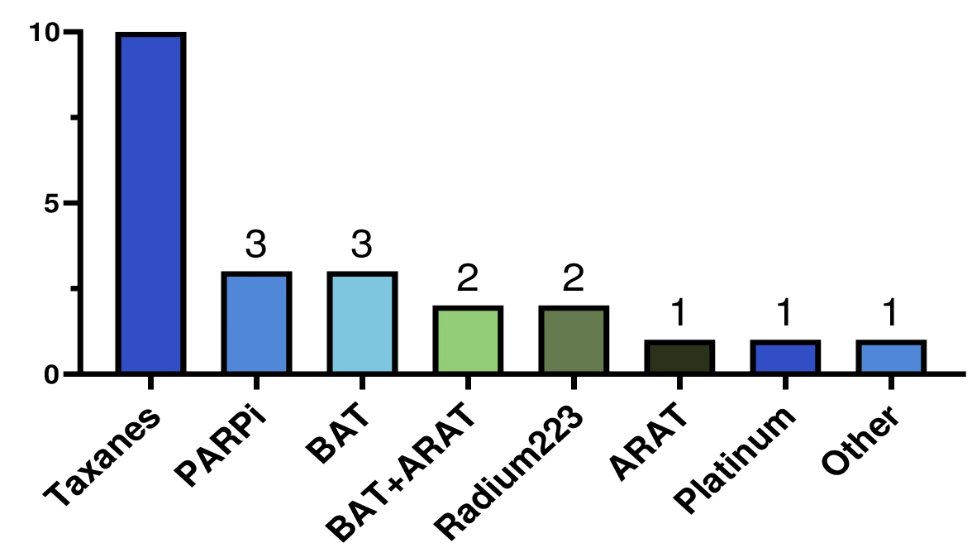
Amongst the patients who received post-PSMA radioligand therapy, 13% achieved a PSA50 response. After a median follow-up of 10 months, the median post-PSMA radioligand therapy specific overall survival in the entire cohort was 11.7 months, with an improvement in outcomes among those having a PSA50 response vs those that did not (HR 0.29, 95% CI 0.09-0.91):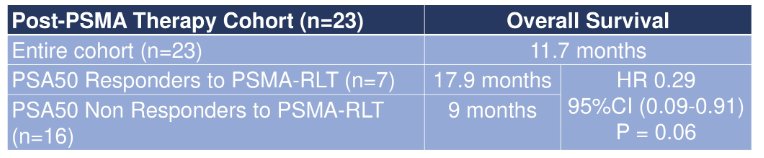
Post-PSMA radioligand therapy specific overall survival was significantly associated with PSA levels at the time of post-PSMA radioligand therapy systemic therapy. The median post-PSMA radioligand therapy specific overall survival for patients with PSA < 100 ng/mL (n = 10), 100 ng/mL ≤ PSA < 1000 ng/mL (n = 8) PSA ≥ 1000 ng/mL (n = 5) was 16.1, 11.7, and 6.1 months respectively (p = 0.015):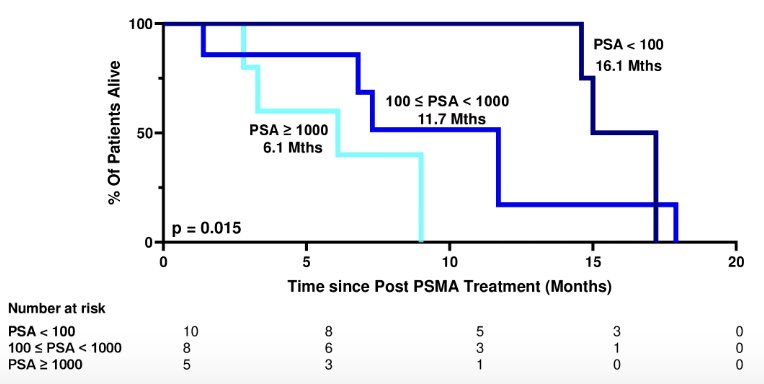
Dr. Labidi concluded this presentation discussing clinical outcomes with systemic therapy given after progression on PSMA radioligand therapy in patients with mCRPC with the following take-home points:
- Responses to next line of therapy after progressing on PSMA radioligand therapy are rare, with PSA level being prognostic in this context
- Response rate to PSMA radioligand therapy was not influenced by prior treatment with taxanes for mCRPC. However, response rates to taxanes was lower when given after PSMA radioligand therapy versus before
- Prospective trials are needed to decide the optimal sequencing of PSMA radioligand therapy and taxanes in mCRPC
Presented by: Soumaya Labidi, MD, Segal Cancer Centre, Jewish General Hospital, Montreal, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Reference:


