(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Frederic Pouliot discussing the prognostic value of FDG, PSMA, and DOTATATE uptake on PET imaging in metastatic castration-resistant prostate cancer (mCRPC). In mCRPC, FDG and PSMA PET/CT are often used in combination for selecting patients for PSMA-radioligand therapy. However, few studies have specifically assessed the prognostic value of FDG+/PSMA- lesions, which exclude patients from PSMA-radioligand therapy. Also, little is known about the significance of somatostatin receptor expression, a potential biomarker of neuroendocrine differentiation of mCRPC, which can be assessed with DOTATATE-PET/CT. 3TMPO is a prospective study in progressing mCRPC patients who were imaged with up to three PET tracers. At the GU ASCO 2024 annual meeting Dr. Pouliot and colleagues reported on patient’s overall survival, with respect to the presence of FDG+/PSMA- and DOTATATE+ lesions.
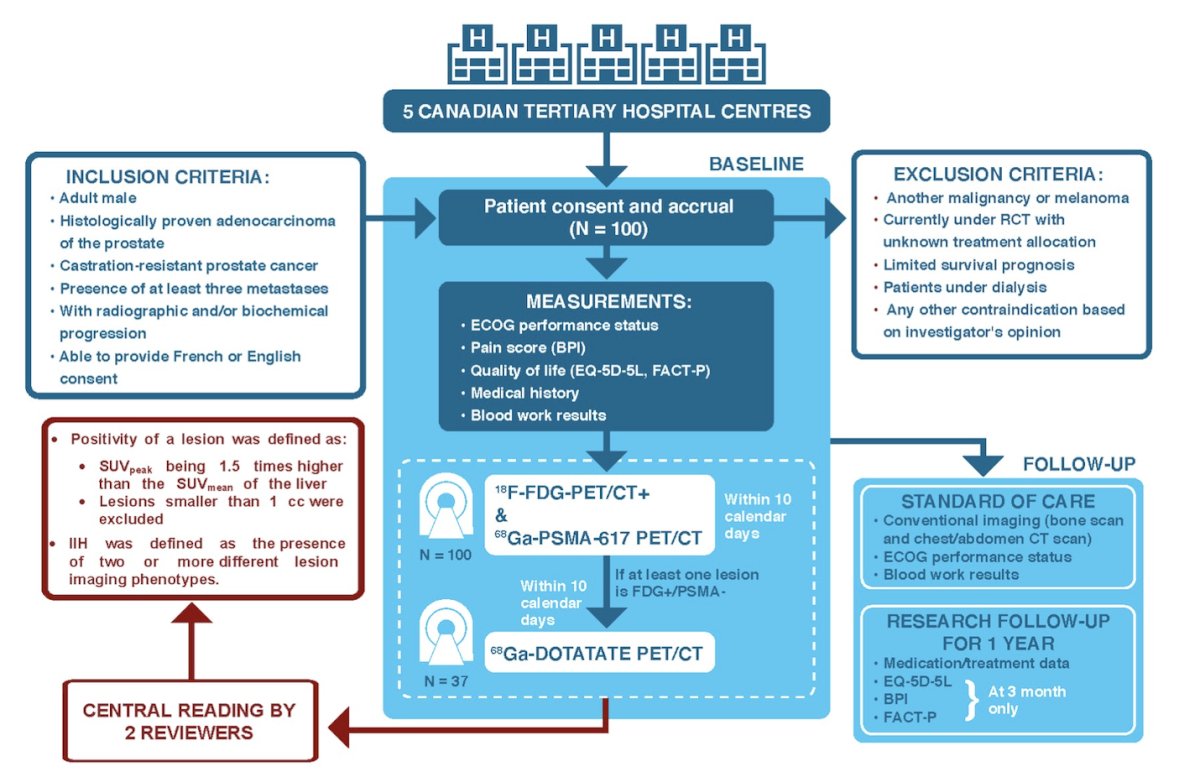
In 3TMPO (NCT04000776),1 all patients had 68Ga-PSMA-617 and 18F-FDG PET/CT scans. A 68Ga-DOTATATE scan was ordered if an FDG+/PSMA- lesion was found. For all tracers, positivity was defined as lesion SUVpeak being 1.5x higher than liver SUVmean. Kaplan-Meier with log-rank test was used to assess the difference in overall survival between groups, and Cox regression model was used to quantify the effect of factors predictive of overall survival. The trial schema is as follows:
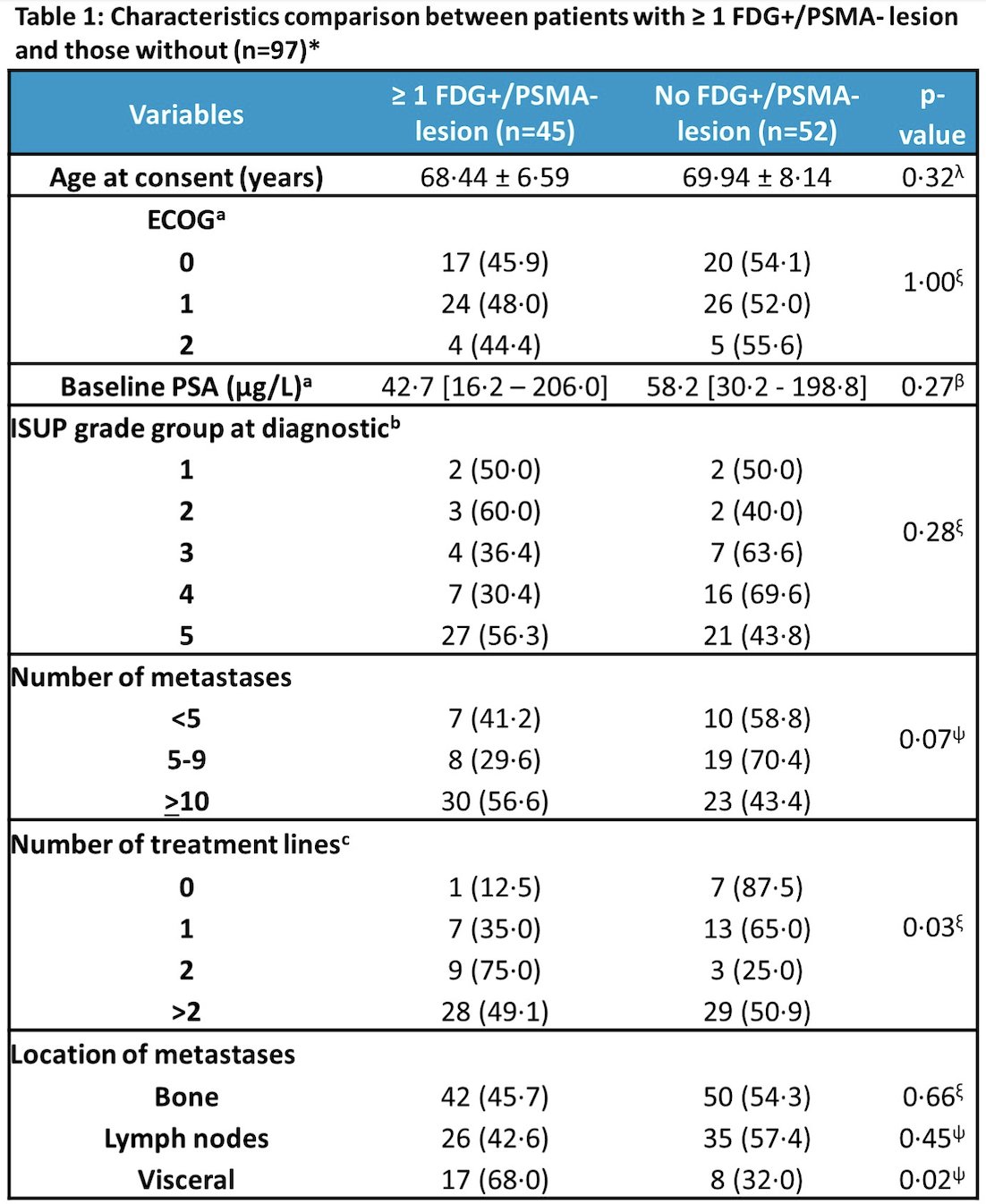
As follows are the characteristics of patients of patients with >=1 FDG+/PSMA- lesion and those without:
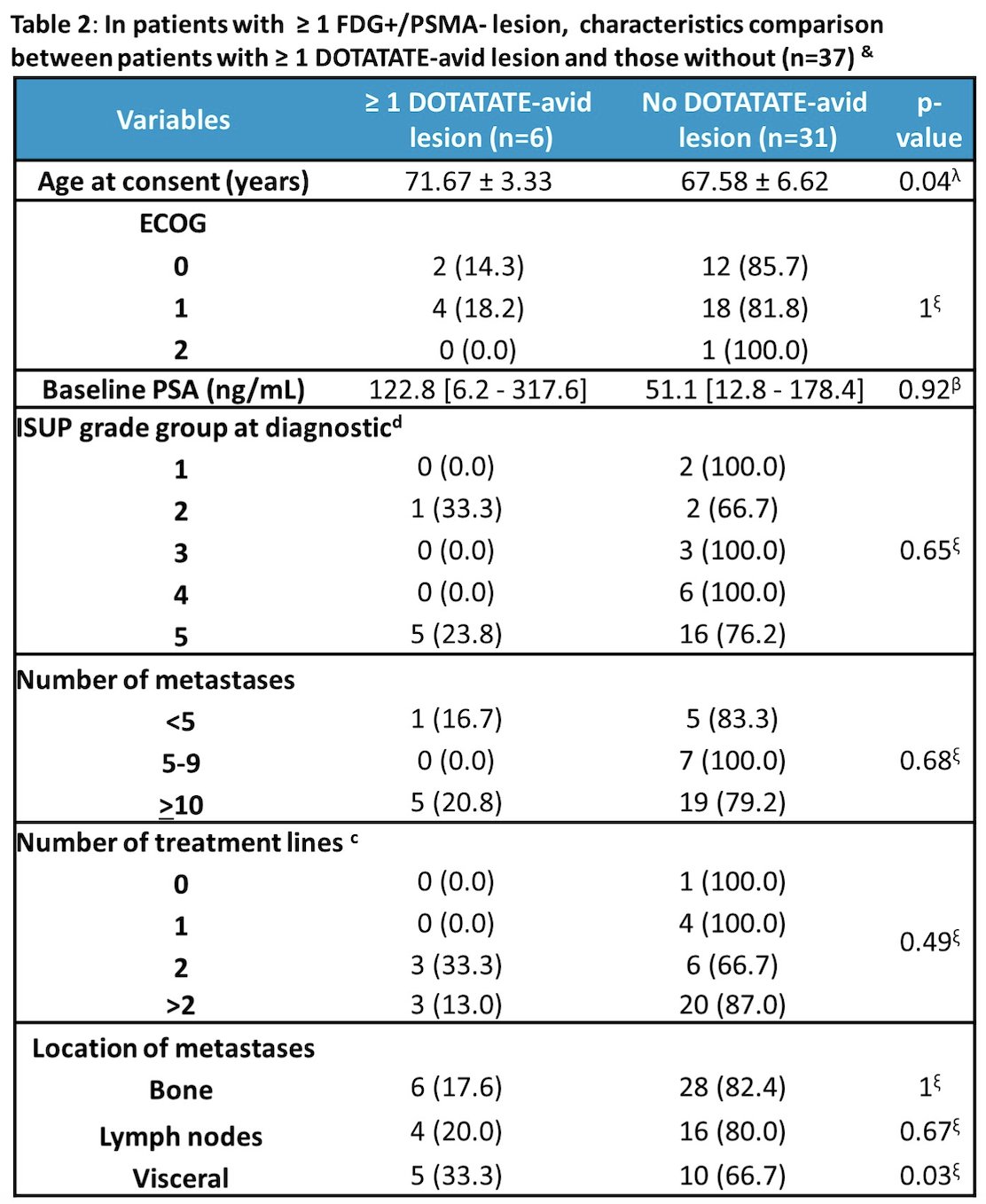
Additionally, the following table highlights the characteristics of patients with >= 1 FDG+/PSMA- lesion and those with >=1 DOTATATE-avid lesions and those without:
The median overall survival of the 98 enrolled patients was 10.2 [95% CI 8.5-11.8] months. At least one FDG+/PSMA- lesion was found in 45 (45.9%) patients and their overall survival was shorter than that of the others: 5.6 [95% CI 4.3-6.9] months vs. not reached (HR 2.75, 95% CI 1.61-4.68; p = 0.0001):
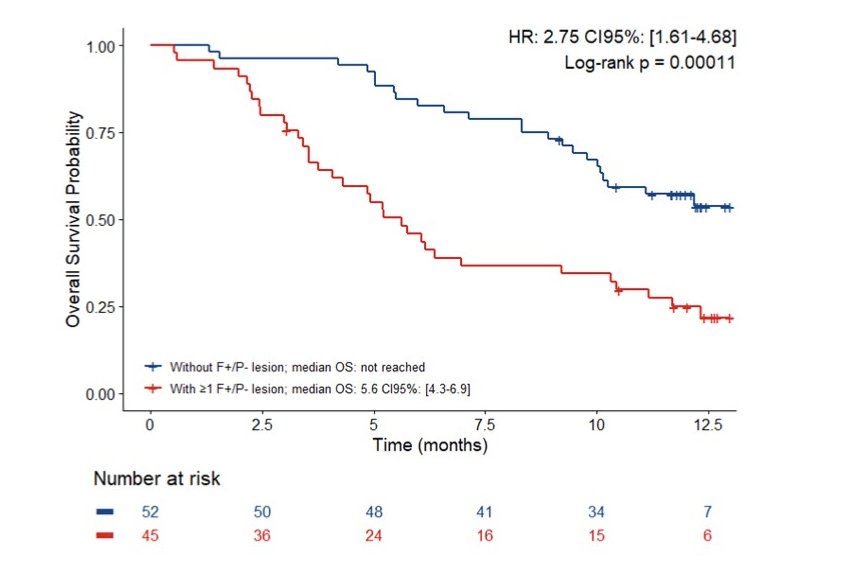
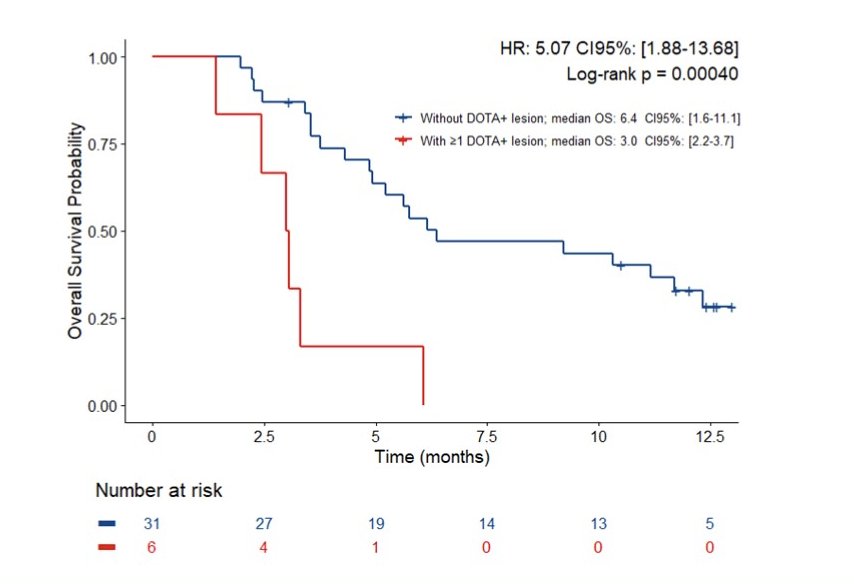
Six (16.2%) of the 37 patients who underwent 68Ga-DOTATATE-PET had ≥1 DOTATATE+ lesion and their overall survival was shorter than that of patients without a DOTATATE+ lesion: 3.0 [95% CI 2.2-3.7] months vs. 6.4 [95% CI 1.6-11.1] months (HR 5.07, 95% CI 1.88-13.68; p = 0.0004):
Characteristics significantly associated with worse overall survival were ECOG, ISUP grade, number of metastases, number of lines of therapy, presence of visceral metastases, FDG and PSMA molecular tumor volumes (p < 0.05). In a multivariate analysis adjusted for the number of metastases and of treatment lines, the presence of an FDG+/PSMA- lesion increased the risk of death (HR 2.4, 95% CI 1.4-4.3, p = 0.002), and this was also significant after adjusting for both PSMA and FDG-molecular tumor volumes (HR 2.9, 95% CI 1.4-5.8, p = 0.003).
Dr. Pouliot concluded his presentation discussing the trial of 3TMPO with the following take-home points:
- mCRPC patients harboring FDG+/PSMA- lesion(s) had a shorter overall survival than those who did not, and their prognosis was even poorer if they also had DOTATATE+ lesion(s)
- Upfront FDG/PSMA-PET followed by DOTATATE-PET might help clinicians to guide patient towards palliative care vs. further systemic therapy, including PSMA-radioligand therapy
Presented by: Frederic Pouliot, MD, PhD, CHU de Québec - Université Laval, Laval, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Pouliot F, Beauregard JM, Saad F, et al. The Triple-Tracer strategy against Metastatic PrOstate cancer (3TMPO) study protocol. BJU Int. 2022 Sep;130(3):314-322.