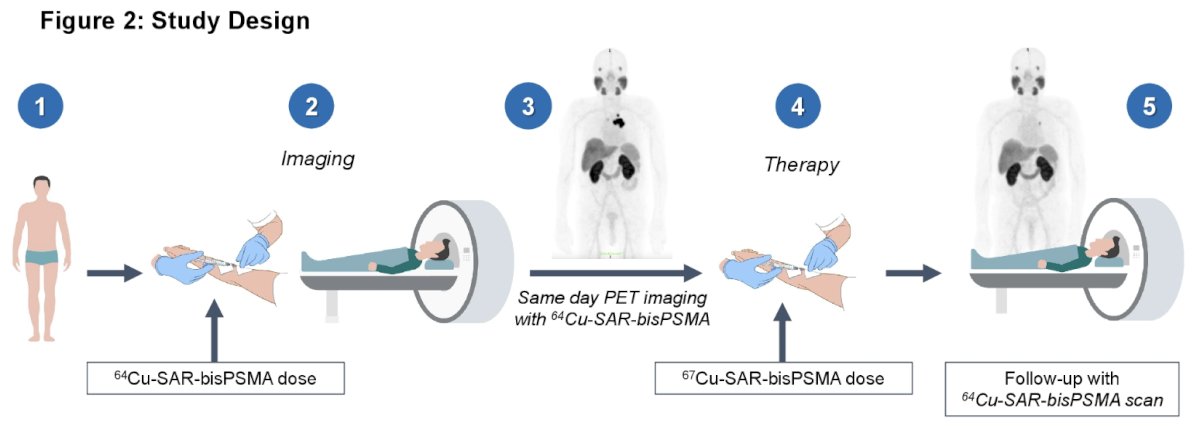
(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer trials in progress poster session. Dr. Geoffrey Johnson presented the study framework of SECuRE, a dose escalation/expansion study to assess the anti-tumor efficacy of 67Cu-SAR-bisPSMA in patients with metastatic castrate-resistant prostate cancer (mCRPC).
Despite recent advances in treatment options across both the castrate sensitive and resistant settings, patients with metastatic prostate cancer still have poor prognoses, with mCRPC patients having an estimated median overall survival of three years in the clinical trial setting.1 As such, the ongoing development of new effective therapies in this space is of utmost importance.
Prostate-specific membrane antigen (PSMA) is expressed in benign and malignant prostate tissues, as well as the luminal surface of salivary/lacrimal glands, small intestine, and renal tubules. The double PSMA binding moiety of SAR-bisPSMA in 64Cu-SAR-bisPSMA (imaging) and 67Cu-SAR-bisPSMA (therapy) may offer advantages compared to currently used single-target PSMA agents.

Clinical data has demonstrated a two- to three-fold higher uptake for 64Cu-SAR-bisPSMA, compared to the single-target PSMA agents. Translational efficacy data of 67Cu-SAR-bisPSMA in mice showed statistically significant tumor growth inhibition compared to the control group, as well as a dose-dependent delay in eventual tumor regression in a prostate cancer xenograft study.2 These results have acted as the rationale for SECuRE, which aims to assess the safety and anti-tumor efficacy of 67Cu-SAR-bisPSMA in mCRPC patients.
SECuRE is a phase 1/2a multi-center, open-label, non-randomized, dose-escalation and cohort expansion study of 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA. Key eligibility criteria are as follows:
- Progressive mCRPC following prior ADT and ≥1 ARPI
- Positive 64Cu-SAR-bisPSMA PET/CT scan, where 64Cu-SAR-bisPSMA uptake (SUVmax) of ≥1 lesion is higher than that of the liver on the 1-hour PET scan
- ≥1 metastatic lesion that is present at screening CT, MRI, or bone scan imaging obtained ≤28 days prior to enrolment
- Eligible for treatment with 67Cu-SAR-bisPSMA
- Prior treatment with systemic radionuclide is allowed after a pre-specified washout period
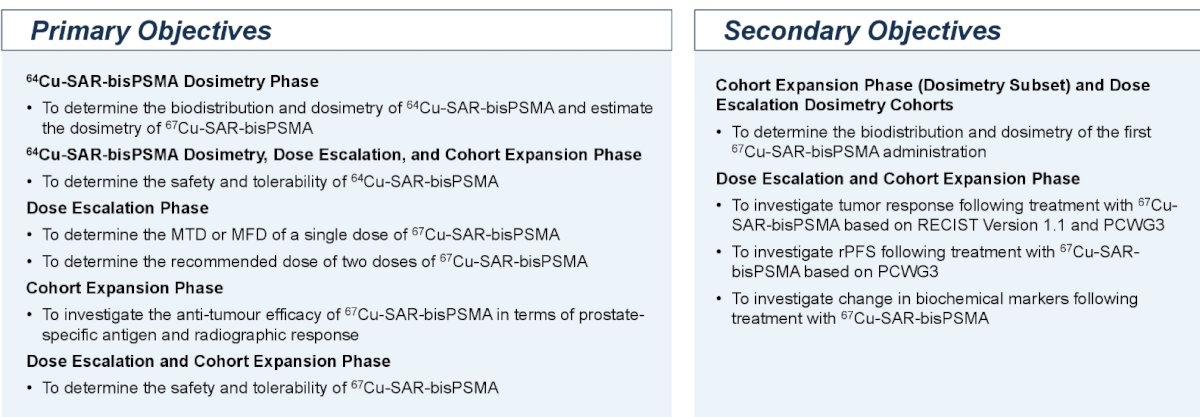
The key primary and secondary objectives are summarized below:
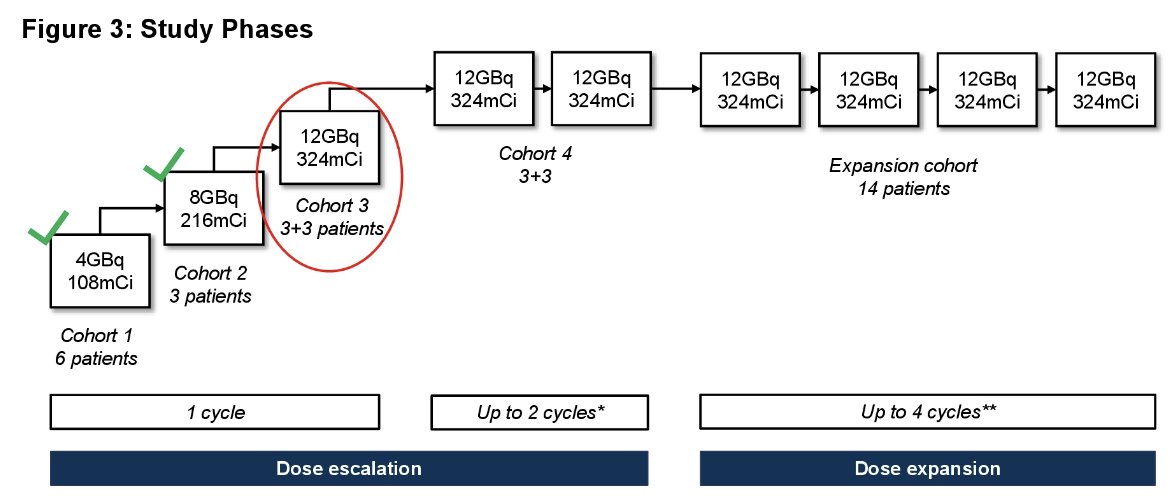
This study is being conducted in 3 phases:
- 64Cu-SAR-bisPSMA Dosimetry Phase (n=6)
- 67Cu-SAR-bisPSMA Dose Escalation Phase (n=up to 24)
- 67Cu-SAR-bisPSMA Cohort Expansion Phase (n=14)
At the time of presentation, no dose limiting toxicities have been observed in cohorts 1, 2, and the first three patients enrolled in cohort 3. In the United States, four sites are active with additional sites in planning. Additional sites in the US and Australia are currently in start-up.
Presented by: Geoffrey Johnson, MD, PhD, Associate Professor, Department of Radiology, Mayo Clinic Rochester, Rochester, MN
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- McInnes LE, Cullinane C, Roselt PD, et al. Therapeutic Efficacy of a Bivalent Inhibitor of Prostate-Specific Membrane Antigen Labeled with 67Cu. J Nucl Med. 2021;62(6):829-832.