(UroToday.com) The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Dr. Evan Yu discussing the trial design of KEYNOTE-365 Cohort J, a phase 1b/2 study of pembrolizumab plus belzutifan and belzutifan alone in patients with docetaxel-treated metastatic castration-resistant prostate cancer (mCRPC). Treatment options are needed for patients with mCRPC, particularly for patients whose disease progresses after docetaxel. Hypoxia-inducible factor–2α (HIF-2α) is a transcription factor established as an oncogenic driver in clear cell renal cell carcinoma. Expression of HIF-2α in prostatectomy specimens has been associated with tumor volume and large tumors more often exhibited higher levels of HIF-2α expression. The first in class selective HIF-2α inhibitor, belzutifan, blocks the heterodimerization of HIF-2α with HIF-1β, resulting in downregulation of oncogenic pathways, and has demonstrated antitumor activity and manageable safety in patients with clear cell renal cell carcinoma.1 Furthermore, the anti–PD-1 antibody pembrolizumab has been shown to have activity as monotherapy and combination therapy in patients with mCRPC. Therefore, the combination of pembrolizumab and belzutifan may provide additional benefit for mCRPC. Cohort J of the multicohort, open-label, phase 1b/2 KEYNOTE-365 (NCT02861573) study will evaluate the safety and efficacy of belzutifan alone (arm J1) and in combination with pembrolizumab (arm J2) in patients with mCRPC who previously received docetaxel.
Eligible patients must be aged ≥18 years, have histologically confirmed adenocarcinoma of the prostate without small cell histology, have an ECOG performance status of 0 or 1, and have received docetaxel for mCRPC. Prior treatment with 1 other chemotherapy and ≤2 second-generation hormonal manipulations are allowed. Approximately 20 patients will receive belzutifan monotherapy 120 mg orally daily. If an efficacy signal is detected in the monotherapy arm, up to 180 patients will be randomly assigned 1:1 to receive pembrolizumab 200 mg IV every 3 weeks + belzutifan 120 mg orally daily or belzutifan alone in an expansion phase. The trial design for KEYNOTE-365 Cohort J is as follows:
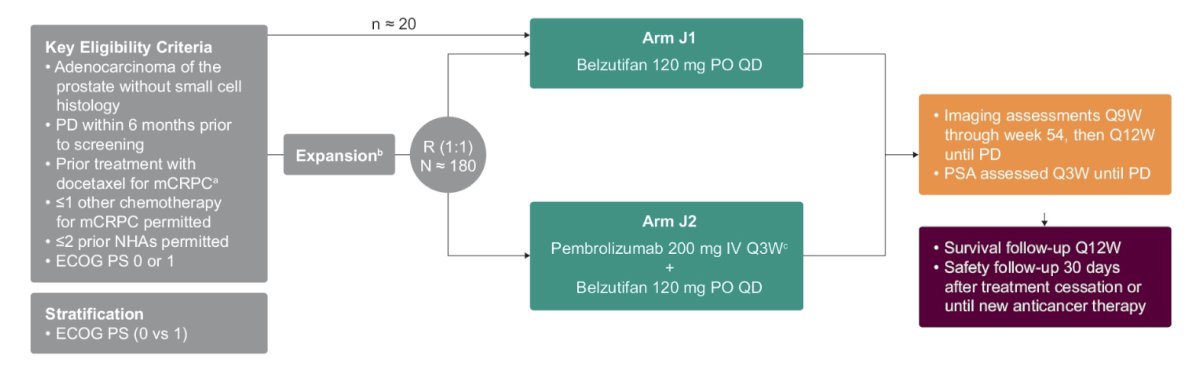
Randomization for the expansion phase will be stratified by ECOG PS (0 vs 1). Imaging assessments (CT/MRI, and bone scans) will be performed every 9 weeks through week 54 and every 12 weeks thereafter, at treatment discontinuation, and during follow-up. PSA will be measured every 3 weeks until progression. The full assessment and follow-up is as follows:
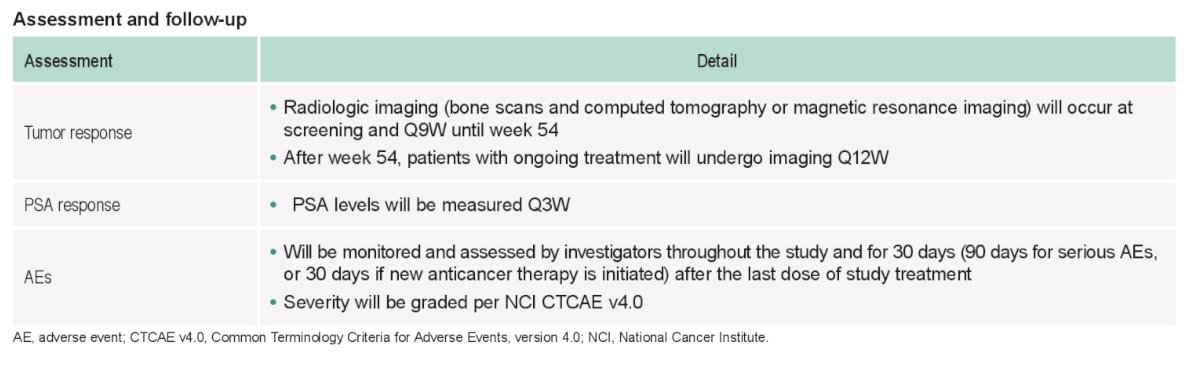
Adverse events will be monitored and assessed by investigators using CTCAE v4.0 throughout the study and for 30 days (90 days for serious adverse events, or 30 days if new anticancer therapy is initiated, whichever occurs first) after the last dose of study treatment.
- Safety and tolerability
- PSA response rate (PSA decline ≥50%)
- Objective response rate per RECIST v1.1 by blinded independent central review
Secondary end points include:
- Time to PSA progression
- Objective response rate
- rPFS per PCWG3-modified RECIST v1.1
- Duration of response
- Disease control rate per RECIST v1.1 by blinded independent central review
- Overall survival
Efficacy and safety will be analyzed in all allocated patients who received ≥1 dose of study treatment, and no formal hypothesis testing will be performed. Enrollment for this study is currently ongoing in the following countries:
Clinical trial information: NCT02861573.
Presented by: Evan Y. Yu, MD, University of Washington, Seattle, WA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


