(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Stephen Freedland presented the results of a real-world analysis of treatment patterns for patients with high-risk, biochemically recurrent, non-metastatic castration-sensitive prostate cancer (nmCSPC).
To date, there is limited level one clinical evidence regarding the treatment of nmCSPC patients with biochemical recurrence (BCR). Therefore, such patients generally receive a treatment strategy based on risk stratification. Recent data suggest that patients with nmCSPC and BCR who are identified as being at high risk of developing metastases may benefit from earlier treatment intensification.1,2 Accordingly, Dr. Freedland and colleagues sought to analyze the real-world 1st line treatment patterns following an nmCSPC diagnosis with high-risk BCR, using data from the Adelphi Real World (ARW) nmCSPC retrospective survey.
United States urologists and radiation oncologists completed the ARW nmCSPC retrospective survey. For this survey, physicians extracted data from medical charts for their last six to ten patients diagnosed with nmCSPC and high-risk BCR, between January 2021 and November 2023. The patient survey inclusion criteria were as follows:
- Age ≥18 years at nmCSPC diagnosis
- Diagnosed with nmCSPC with BCR after definitive therapy or PSA recurrence; BCR confirmed during or after January 2021
- Classified as high-risk BCR as understood/dictated by the consulting physician
- Treated or managed by the physician for nmCSPC with high-risk BCR
- Not involved in a clinical trial as part of their nmCSPC treatment management
Data were descriptively analyzed for 1st line treatment received after diagnosis of high-risk BCR for the total patient sample and subgroups of interest (patients with and without PSA doubling time ≤9 months, by treating physician specialty, and by physician practice setting). Physicians documented patients that did not receive 1st line treatment during the data collection period as being “on observation”. The follow up period was defined as the time from high-risk biochemical recurrence diagnosis to data collection.
Aggregated patient-level data were analyzed for 535 patients with nmCSPC and high-risk BCR. Overall, 80% of patients were treated by urologists and 20% by radiation oncologists. 56% were treated in a community hospital/center. The median time between the date of high-risk BCR diagnosis and data collection was 286 days, and the median time of data collection since the start of 1st line treatment was 234 days. The median time between high-risk BCR diagnosis and initiation of 1st line treatment was 21 days. For patients on observation alone, median time of data collection after high-risk BCR diagnosis was 201 days.
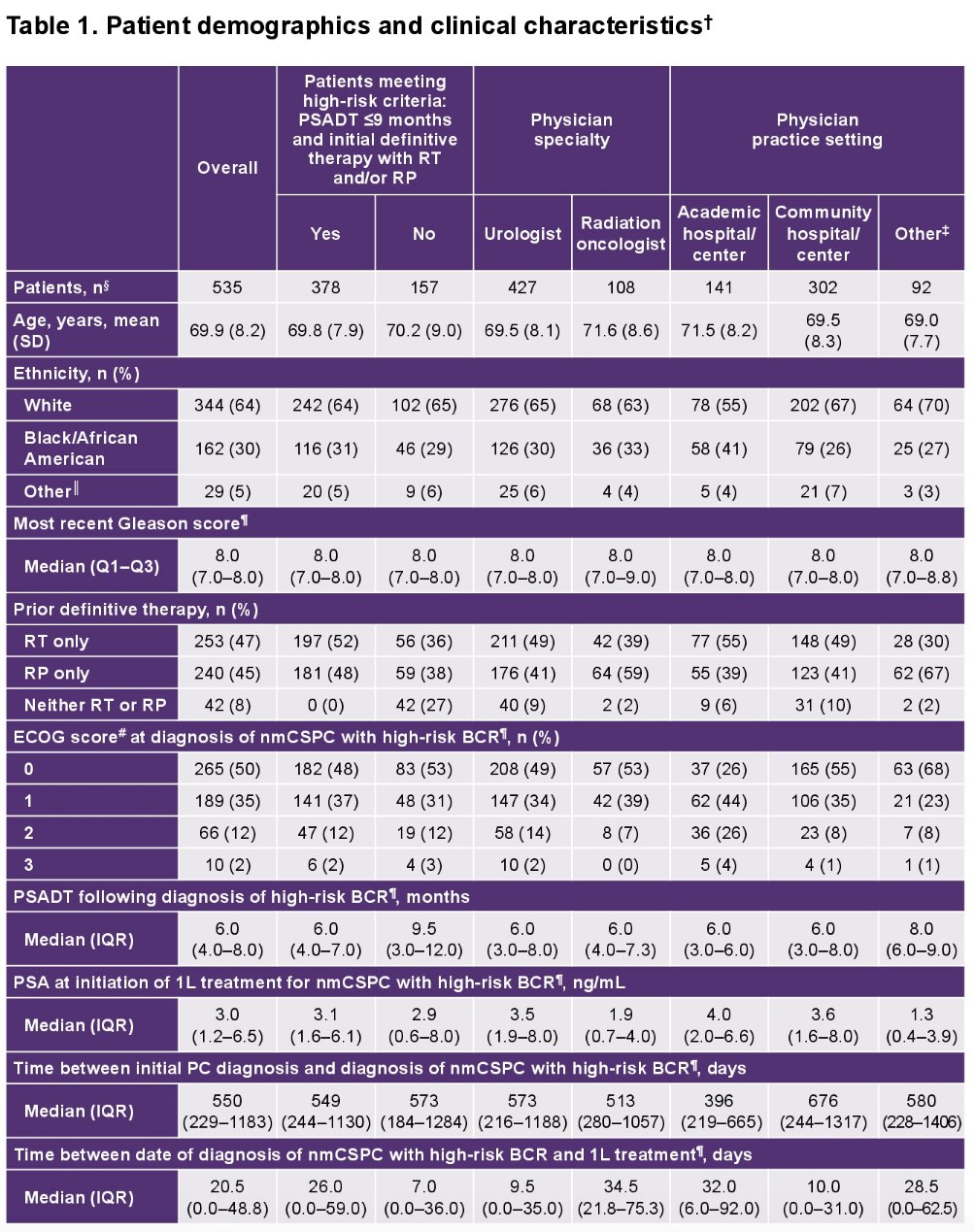
The most common 1st line treatments were ADT alone, radiation therapy, novel hormonal therapy + ADT, and observation alone.
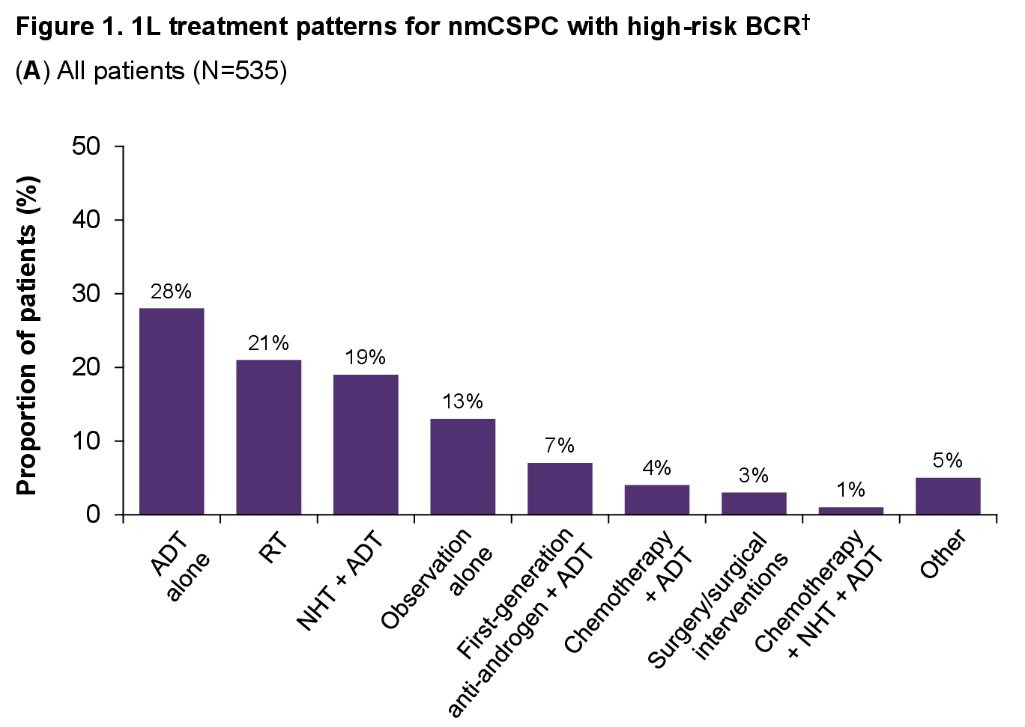
Few numerical differences were seen in treatment patterns when patients were stratified by specific high-risk criteria or by physician practice setting.
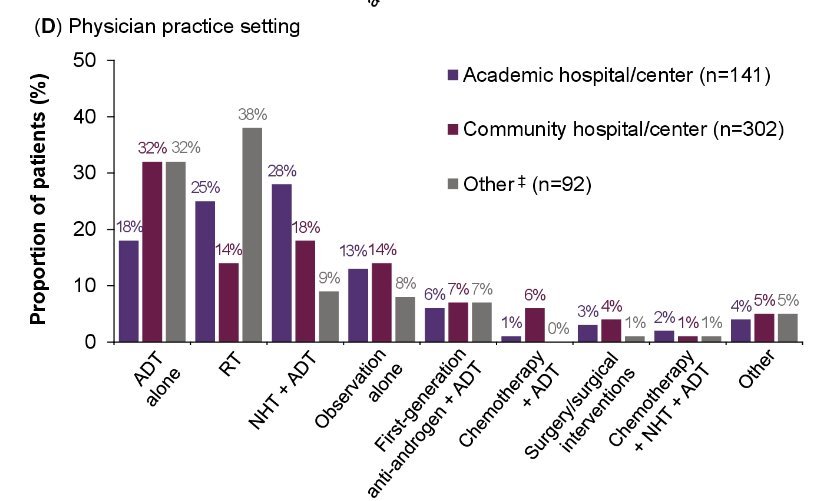
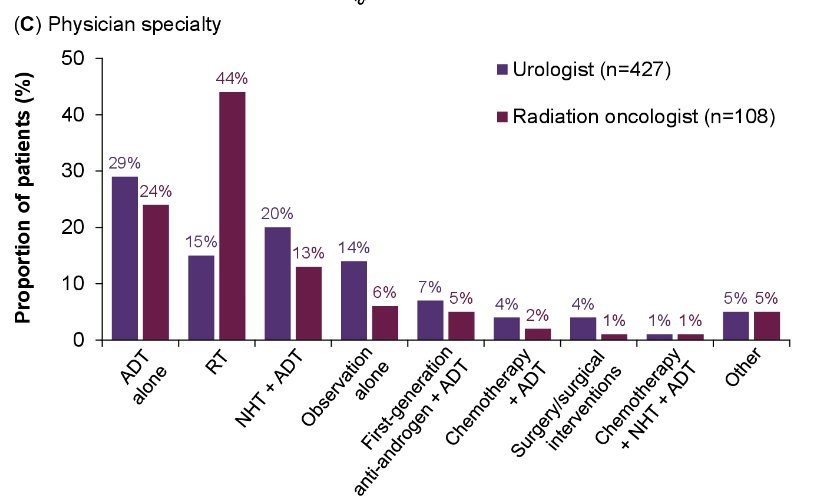
However, differences were apparent for patients treated by urologists and radiation oncologists, with radiation oncologists recommending radiotherapy for 44% of such patients (versus 15% for urologist-managed patients).
When patients received ADT, either alone or in combination with other treatments, most patients received ADT as continuous (79%) rather than intermittent administration (21%). Results were generally similar across subgroups; however, a higher proportion of patients treated by radiation oncologists received intermittent ADT (35%) versus patients treated by urologists (19%).
Dr. Freedland concluded that 1st line treatments were typically initiated rapidly following high-risk BCR nmCSPC diagnosis, with only 15% of patients receiving observation alone during available follow-up.
Presented by: Stephen Freedland, MD, Professor, Department of Urology, Cedars Sinai Hospital, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Virgo KS, Rumble RB, Talcott J. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J Clin Oncol. 2023;41(20):3652-6.
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023;389(16):1453-65.


